|
|
|
Indian Pediatr 2021;58:253-258 |
 |
Fortification of Human
Milk With Infant Formula for Very Low Birth Weight Preterm
Infants: A Systematic Review
|
|
Manish Kumar, 1
Jaya Upadhyay2
and Sriparna Basu2
From Departments of 1Pediatrics and 2Neonatology,
All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India.
Correspondence to: Sriparna Basu, Department of Neonatology, All
India Institute of Medical Sciences, Rishikesh,
Uttarakhand -249203, India.
Email:
[email protected]
Received: July 10, 2019;
Initial review: November 05, 2019;
Accepted: October 29, 2020.
PROSPERO Registration Number: CRD42019138122
Early online: January 02, 2021;
PII: S097475591600277
|
Background: Off-label fortification of expressed
human milk (HM) with infant milk formula (IMF) is common in developing
countries, though its benefits and safety are unclear.
Objective: To study the effects of IMF
fortification of HM on growth of very low birth weight (VLBW) preterm
infants.
Design: Systematic review and meta-analysis of
randomized and quasi-randomized controlled trials (RCTs).
Data sources and selection criteria: MEDLINE,
EMBASE, CINAHL, CENTRAL and other databases were searched for articles
published in English language from inception to December 2019,
evaluating the effects of HM fortified with IMF as intervention,
compared to unfortified HM or HM fortified with human milk fortifier
(HMF).
Participants: Five RCTs including 423 VLBW
preterm infants.
Intervention: Feeding with HM fortified with IMF
compared to unfortified or HMF-fortified HM.
Outcome measures: Primary outcome measure was
assessment of growth as weight, length and head circumference (HC) gain
velocity. Secondary outcome measures were incidences of feed intolerance
(FI), necrotizing enterocolitis (NEC), time to reach full feeds,
concentration of nutritional biomarkers, duration of hospital-stay and
cost of intervention.
Results: Of the five studies included in the
review, pooled effects regarding weight gain velocity (SMD 0.27
g/day; 95% CI 0.08 to 0.62), length gain (MD 0.07cm/week; 95% CI 0.02 to
0.16) and HC gain (MD 0.05 cm/wk; 95% CI 0.01 to 0.11), were not
statistically significant. Sensitivity analysis by pooling studies using
unfortified milk as comparator yielded a statistically significant
result for all growth parameters. Risk of FI or NEC was comparable.
Length of hospitalstay was reduced in th intervention group.
Conclusions: A very-low quality evidence
suggested that IMF fortification of HM is superior to unfortified milk
and may be a safe alternative for HMF for short term growth of VLBW
preterm infants.
Keywords: Human milk, Human milk fortification, Preterm, Very
low birth weight infant
|
|
E very year,
approximately14.9 million neonates, representing a birth rate of
11.1%, are born preterm, globally [1]. Though substantial
advancement in medical care has led to an improved survival of
preterm infants [2], significant morbidity during the hospital
stay and adverse long-term neurological consequences remain
major areas of concern.
Deprived of the third trimester accretion of
macro and micronutrients, along with the inability to meet the
increased postnatal demand due to prematurity-related illnesses
and poor nutritional intake, more than half of these infants
have extra-uterine growth restriction, which in turn has
long-term adverse cardiovascular and metabolic consequences
[3,4]. Nutritional optimization is considered vital for
survival, growth, and improved neurodevelopmental outcome [5-8].
Though breastmilk is the nutrition of choice
for very low birth weight (VLBW) preterm neonates [9], exclusive
human milk (HM) feeding, does not meet their nutritional targets
[10,11]. Moreover, after two weeks, the protein content of milk
of mothers delivering preterm decreases further [12].
Multi-nutrient fortification of HM results in increased rate of
gain in weight, length and head circumference of VLBW preterm
infants [13-16].
Unfortunately, in low- and middle-income
countries (LMICs), the concept of individualized and targeted
fortification is far from implementation. Commercially available
human milk fortifiers (HMF) are low in protein content (<1g/100
mL) and expensive, prohibiting routine supplementation [17]. An
alternative and more econo-mical strategy, commonly employed
off-label in various neonatal units, is to enrich EBM by adding
infant milk formula (IMF) to achieve the required level of
protein for improved growth outcomes [18-22]. However, IMF
fortification may result in increased osmolarity, non-uniform
protein content and risk of contamination leading to feeding
intolerance (FI), sepsis and necrotizing enterocolitis (NEC). In
addition, the quantity needed for optimum fortification and
measuring technique is not validated.
This systematic review intended to evaluate
the role of fortification of HM with IMF for growth in VLBW
preterm infants.
Methods
This systematic review and meta-analysis was
conducted in accordance to PRISMA guidelines [23].
Search strategy and search criteria: All
authors independently searched the databases including PubMed,
Embase, Cochrane Central Register of Controlled Trials, other
clinical trial registries, Google Scholar, Scopus, Web of
Science and hand searching of conference proceedings from
inception to December 2019 for peer-reviewed publications in
English language. The electronic search strategy included a
combination of keywords along with their representative medical
subjects headings (MeSH) terms. Details of search strategy are
provided in Web Appendix 1. Reference list of all
articles whose full texts were screened, was also checked to
find additional articles.
We included randomized or quasi-randomized
controlled trials (RCT) evaluating the effects of HM fortified
with IMF as intervention, compared to unforti-fied or
HMF-fortified HM on growth rate, duration of hospital-stay and
other clinically relevant outcomes in VLBW preterm infants.
Non-English publications were excluded.
The primary outcome was assessment of
velocity of gain in weight, length, and head circumference (HC).
Secondary outcomes were duration of hospital stay, incidences of
FI and NEC, time to reach full feeds, concen-tration of
nutritional biomarkers (calcium, phosphorous, blood urea
nitrogen, prealbumin, albumin, alkaline phos-phatase) and cost
of intervention.
Data extraction and quality assessment:
Two authors independently extracted data using a pre-designed
pro-forma. Disagreement, if any, was resolved by discussion with
third author. Study details including location and year of
study, number of infants and their characteristics, details of
feeding including fortification and outcomes relevant to the
study were noted. Quality of studies were assessed independently
by all authors, for each study, using the risk of bias (ROB)
criteria outlined in the Cochrane Handbook for Systematic
Reviews of Interventions [24] in the domains of random sequence
generation, allocation concealment, blinding of partici-pants
and personnel, blinding of outcome assessment, incomplete
outcome data, selective outcome reporting, and other bias.
Statistical analysis: Statistical
analysis was performed using Review Manager version 5.4 (The
Cochrane Colla-boration, 2020). Out-come variables were
calculated as risk ratio (RR) with 95% confidence interval (CI)
for dichotomous data and mean differences (MD) with 95% CI for
continuous data. Standardized mean differences (SMDs) were
calculated where outcomes had different measurement instruments.
Studies reporting dispersion of outcomes in range was converted
to standard deviation using established mathematical models
[25]. Results were pooled using either fixed or random effects
model based on hetero-geneity which was assessed using the I²
statistic. Grading of recommendations assessment, development
and evaluation (GRADE) approach [26] was applied to assess the
quality of evidence for predefined outcomes.
RESULTS
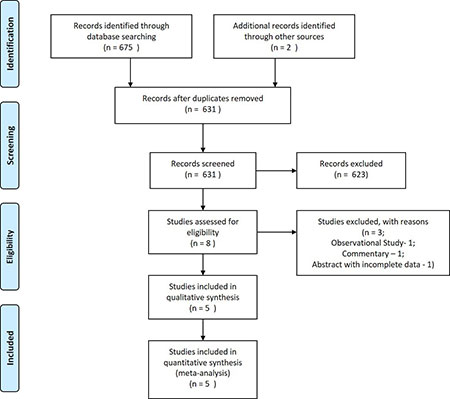
Screening and inclusion of studies are
summarized in Fig.1. Four full-text articles
[18-21] and one abstract [22] were selected for this systematic
review including a total of 423 VLBW preterm infants.
 |
|
Fig. 1 PRISMA flow diagram.
|
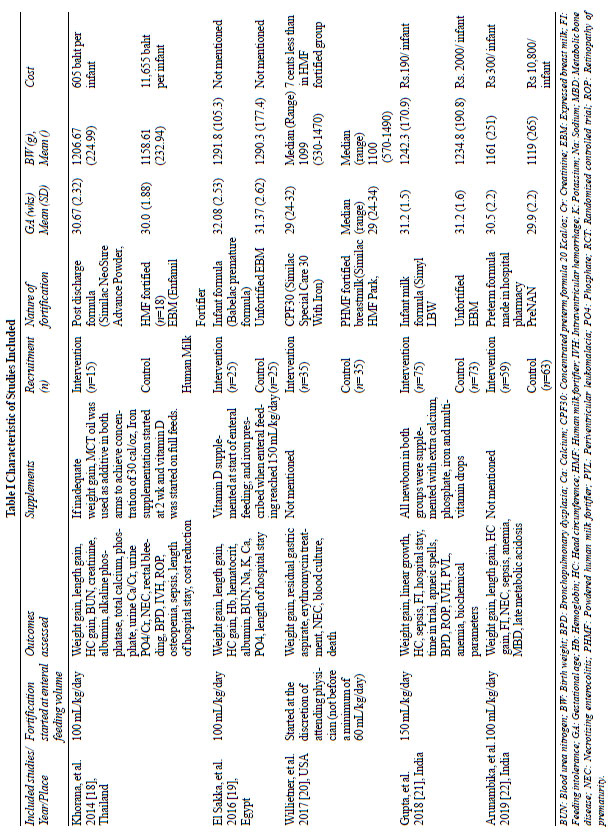
The characteristics of included studies are
summarized in Table 1. The birth weight of the preterm
VLBW infants included in the studies, ranged from 500g to 1499g.
Fortification of HM with IMF was the intervention in all five
trials. The time to start fortification varied from 100 mL/kg/d
[18,19,22] to150 mL/kg/d of enteral feed [21]. Willeitner, et
al. [20] introduced fortification as early as at 60 mL/kg/d, at
the discretion of the treating team. In three studies [18,20,22]
the comparator was HMF, while other two studies [19,21] used
unfortified HM. Web Fig. 1 depicts ROB graph
summarizing each ROB item as percentage across all studies while
Web Fig. 2 summarizes ROB for each included study.
 |
| |
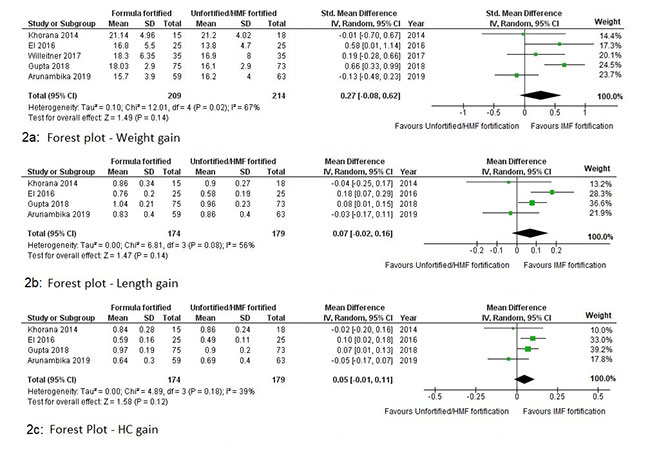
All included studies evaluated weight gain
velocity as primary outcome. Four studies [19,20-22], described
weight gain velocity in terms of g/kg/day, while Khorana, et al.
[18] reported weight gain as g/day. Overall, pooled effects of
all five studies on weight gain velocity was statistically not
significant (SMD 0.27 g/kg/day; 95% CI: -0.08 to 0.62) (Fig.
2a). Sensitivity analysis was done due to difference in
comparators. IMF fortification was found to cause a
statistically significant increase in the rate of weight gain
(MD 02.03 g/day; 95% CI: 1.15 to 2.92) compared to unfortified
HM. Using HMF as comparator, SMD of weight gain velocity was
similar (SMD -0.01 g/day; 95% CI: -0.27 to 0.25).
 |
|
Fig. 2 Forest plot showing
meta-analysis of the effect of infant milk fortification
on the velocity of weight gain (2a), length gain (2b)
and head circumference (HC) gain (2c).
|
Four studies [18,19,21,22] with 353
participants reported data regarding rate of increase in length
and HC. The pooled effect with respect to velocity of gain in
length was not statistically significant (MD 0.07 cm/week; 95%
CI: -0.02 to 0.16) (Fig. 2b). On sensitivity
analysis, when compared to unfortified HM, IMF fortification
resulted in significantly higher rate of gain (MD 0.12 cm/week;
95% CI: 0.02 to 0.22), but failed to show difference when
compared with HMF (MD -0.03 cm/week; 95% CI: -0.15 to 0.08).
Similarly, the pooled effect with respect to velocity of gain in
HC was not statistically significant (MD 0.05 cm/week; 95% CI:
-0.01 to 0.11) (Fig. 2c). On sensitivity analysis,
when compared to unfortified HM, IMF fortification resulted in
significantly higher rate of gain (MD 0.08 cm/week; 95% CI: 0.03
to 0.13), but failed to show difference when compared with HMF
(MD -0.04 cm/week; 95% CI: -0.14 to 0.06).
FI, reported in two studies [19,21] (n=208),
showed no difference in risk between IMF and HMF fortification
versus no fortification of HM (RR 2.29; 95% CI: 0.61to 8.59) (Web
Fig. 3a). Though HMF fortification showed apparently
higher rates of NEC [18,20], the RR was not statistically
significant for either suspected NEC (RR 0.37; 95% CI: 0.07 to
1.95) (Web Fig. 3b) or confirmed NEC (RR 0.25; 95%
CI: 0.04 to 1.39) (Web Fig. 3c).
Three studies [18,19,21] including 231
participants, showed that the length of hospital stay of
neonates with IMF was significantly reduced (MD -4.38 days; 95%
CI: -7.39 to -1.37) (Web Fig. 3d). Two studies [18,19] (n=83)
found no significant difference with respect to time to achieve
full enteral feeding, between those receiving formula fortified
HM and those on either HMF fortified or unfortified HM. (Web
Fig. 3e). Effect of fortification on nutritional biomarkers
were reported by two studies [18,21]. No significant effect on
BUN nor albumin levels was observed (Web Fig.3f, 3g).
Though four of the studies favored IMF
intervention in terms of cost, this economical aspect was not
studied as an outcome in any of them. The data presentation was
not uniform and therefore, could not be pooled.
The quality of evidence pooled from included
studies was assessed using GRADE approach and summary of
findings table was generated on GRADE pro GDT software (Evidence
Prime Inc.) (Web Appendix 2).
DISCUSSION
This systematic review and meta-analysis of
five RCTs, including a total of 423 VLBW preterm infants, did
not show any significant benefit of IMF fortification of HM over
combined HMF fortification/no fortification, on growth velocity,
with respect to weight, length and HC. On sensitivity analysis
for the same parameters, IMF and HMF fortifications were
comparable, whereas IMF fortification was significantly better
than unfortified HM, quality of evidence (QOE) being very low.
No significant difference was noted in the incidences of FI/NEC
and levels of nutritional biomarkers like BUN and albumin (QOE:
very low). Pooled data from three trials, showed a significant
reduction in duration of hospital stay favoring IMF
fortification (QOE, very low). This reduction was probably
because the comparator in two of these studies was unfortified
HM.
There are several limitations in the included
trials. The study by El Sakka, et al. [19] was quasi-randomized
with an unclear methodology. Still this study was included as
its outcome measures met our inclusion criteria. The gestational
age varied among the studies, with one trial [21] excluding late
preterm infants. No data were available regarding long term
growth and developmental outcome. Formulas and HMFs preparations
used were from different manufacturers, though the protein and
energy content were similar. Another area of discrepancy was
non-uniform timing of initiation of fortification in included
trials, which might have affected growth. The most important
concern for implementation of IMF fortification in routine
practice is increase in osmolarity with risk of FI and NEC. Only
one trial [21] measured osmolarity of HM after IMF fortification
and found it below 400 mOsm/L, the recommended upper safety
limit of American Academy of Pediatrics [27]. Though no
difference in the incidences of FI and NEC was noted, none of
the studies was adequately powered to detect the difference.
None of the trials had individualized the fortification by
analysis of HM macronutrients. IMF measurement technique for
fortification was described by only one study [22].
A relatively limited number of studies, with
high ROB and statistical heterogeneity in this systematic review
limit the generalizability of this meta-analysis. Variability in
the time of initiation of feed, the maximum feeding volume and
continuation of IMF as ‘bridge feeding’ when EBM was unavailable
[20] probably limited the impact of the intervention on growth
outcomes. Further, subgroup analysis based on gestation or birth
weight could not be done because of unavailability of raw data.
Not all biomarkers of nutrition could be evaluated due to lack
of measured values. Data regarding cost could not be pooled as
there was no uniformity in presentation.
To summarize, a very-low quality evidence
suggests that IMF fortification of HM is superior to unfortified
HM and may be a safe alternative for bovine HMFs for short term
growth of VLBW preterm infants, especially in resource-limited
settings. Larger well-designed studies with strict monitoring of
complications including NEC with a focus on long-term outcomes
are needed.
Acknowledgement: Dr. Poonam Singh
for assistance in revising the manuscript.
Contributors: MK:
conceptualized the review, literature search, data analysis and
manuscript writing; JU: literature search, data analysis and
manuscript writing; SB: conceptualized the review, literature
search, data analysis and manuscript writing.
Funding: None; Competing interest:
None stated.
REFERENCES
1. Blencowe H, Cousens S, Chou D, et al. Born
too soon: The global epidemiology of 15 million preterm births.
Reprod Health. 2013;10:S2.
2. Helenius K, Sjors G, Shah PS, et al.
Survival in very preterm infants: An international comparison of
10 national neonatal networks. Pediatrics. 2017; 140:e20171264.
3. Horbar JD, Ehrenkranz RA, Badger GJ, et
al. Weight growth velocity and postnatal growth failure in
infants 501 to 1500 grams: 2000-2013. Pediatrics.
2015;136:e84-92.
4. Embleton ND. Early nutrition and later
outcomes in preterm infants. World Rev Nutr
Diet. 2013;106:26-32.
5. Ong KK, Kennedy K, Castañeda-Gutiérrez E,
et al. Postnatal growth in preterm infants and later health
outcomes: a systematic review. Acta Paediatr. 2015; 104:974-86.
6. Brandt I, Sticker EJ, Lentze MJ. Catch-up
growth of head circumference of very low birth weight, small for
gestational age preterm infants and mental development to
adulthood. J Pediatr. 2003;142:463-70.
7. Leppänen M, Lapinleimu H, Lind A, et al;
PIPARI Study Group. Antenatal and postnatal growth and 5-year
cognitive outcome in very preterm infants. Pediatrics. 2014;
133:63-70.
8. Arslanoglu S, Boquien CY, King C, et al.
Fortification of Human Milk for Preterm Infants: Update and
Recommendations of the European Milk Bank Association (EMBA)
Working Group on Human Milk Fortification. Front Pediatr.
2019;7: 76.
9. Eidelman AI. Breastfeeding and the use of
human milk: An analysis of the American Academy of Pediatrics
2012 breastfeeding policy statement. Breastfeeding Med. 2012; 7:
323-4.
10. Embleton ND. Optimal protein and energy
intakes in preterm infants. Early Hum Dev. 2007;83:831-7.
11. Agostoni C, Buonocore G, Carnielli VP, et
al; ESPGHAN Committee on Nutrition. Enteral nutrient supply for
pre-term infants: commentary from the European Society of
Paediatric Gastroenterology, Hepatology and Nutrition Committee
on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85-91.
12. Lucas A, Hudson GJ. Preterm milk as a
source of protein for low birth weight infants. Arch Dis Child.
1984;59:831-6.
13. Arslanoglu S, Moro GE, Ziegler EE. The
WAPM working group on nutrition. optimization of human milk
fortifi-cation for preterm infants: New concepts and
recommen-dations. J Perinat Med. 2010;38:233-8.
14. Ziegler EE. Meeting the nutritional needs
of the low-birth-weight infant. Ann Nutr Metab. 2011;58:8-18.
15. Rochow N, Landau-Crangle E, Fusch C.
Challenges in breast milk fortification for preterm infants.
Curr Opin Clin Nutr Metab Care. 2015;18:276-84.
16. Brown JV, Embleton ND, Harding JE,
McGuire W. Multi-nutrient fortification of human milk for
preterm infants. Cochrane Database Syst Rev. 2016;5:CD000343.
17. Kler N, Thakur A, Modi M, et al. Human
Milk Fortification in India. Nestle Nutr Inst Workshop Ser.
2015;81:145-51.
18. Khorana M, Jiamsajjamongkhon C. Pilot
study on growth parameters and nutritional biochemical markers
in very low birth weight preterm infants fed human milk
fortified with either human milk fortifier or post discharge
formula. J Med Assoc Thai. 2014; 97:S164-75.
19. El Sakka A, El Shimi MS, Salama K, Fayez
H. Post discharge formula fortification of maternal human milk
of very low birth weight preterm infants: An introduction of a
feeding protocol in a university hospital. Pediatr Rep.
2016;8:6632.
20. Willeitner A, Anderson M, Lewis J. Highly
concentrated preterm formula as an alternative to powdered human
milk fortifier: A randomized controlled trial. J Pediatr Gastro-enterol
Nutr. 2017; 65: 574-8.
21. Gupta V, Rebekah G, Sudhakar Y, Santhanam
S, Kumar M, Thomas N. A randomized controlled trial comparing
the effect of fortification of human milk with an infant formula
powder versus unfortified human milk on the growth
of preterm very low birth weight infants. J Matern Fetal
Neonatal Med. 2020;33:2507-15.
22. Arunambika C, Sharma A, Jeevasankar M.
Comparison of fortification of expressed breast milk with
preterm formula powder and human milk fortifier in preterm very
low birth weight neonates: A randomized, non-inferiority trial
[abstract]. In: Goswami VP, Bharti LK, Chandra USJ, et
al., editors. Abstracts of the 39th Annual Convention of
National Neonatology Forum; 2019 December 12-15; Hyderabad,
India. 2019.p.3.
23. Moher D, Liberati A, Tetzlaff J, Altman
DG. The PRISMA Group. Preferred reporting Items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
2009;6:e1000097.
24. Higgins JPT, Thomas J, Chandler J, et
al., editors. Cochrane Handbook for Systematic Reviews of
Interventions. 2nd ed. Chichester (UK): John Wiley & Sons, 2019.
25. Ramirez A, Charles C. Improving on the
range rule of thumb. Rose-Hulman UMJ. 2012;13:1-13.
26. Schünemann H, Broek J, Guyatt G, Oxman A,
editors. GRADE handbook for grading quality of evidence and
strength of recommendations. Updated October 2013. The GRADE
working group, 2013.
27. Barness LA, Mauer AM, Holliday MA. Commentary
on breast-feeding and infant formulas, including proposed
standards for formulas. Pediatrics. 1976;57:278-85.
|
|
|
 |
|

