|
|
|
Indian Pediatr 2013;50:
283-288 |
 |
Etiology and Outcome of Crescentic
Glomerulonephritis
|
|
Aditi Sinha, Kriti Puri, Pankaj Hari, *Amit Kumar Dinda
and Arvind Bagga
From the Division of Nephrology, Department of Pediatrics, and
*Department of Pathology, All India Institute of Medical Sciences, New
Delhi, India.
Correspondence to: Dr Arvind Bagga, Professor, Division of
Nephrology, Department of Pediatrics, 3053, Teaching Block, All India
Institute of Medical Sciences, New Delhi.
Email: [email protected]
Received: June 07, 2012;
Initial review: June 26, 2012;
Accepted: August 02, 2012.
Published online: 2012, July 5.
PII: S097475591200481
|
Objective: To determine the etiology, course and predictors of
outcome in children with crescentic glomerulonephritis (GN).
Study design: Retrospective, descriptive study.
Setting: Pediatric Nephrology Clinic at a
referral center in Northern India.
Methods: Clinic records of patients aged <18 year
with crescentic GN diagnosed from 2001-2010 and followed at least
12-months were reviewed. Crescentic GN, defined as crescents in
³50%
glomeruli, was classified based on immunofluorescence findings and
serology. Risk factors for renal loss (chronic kidney disease stage 4-5)
were determined.
Results: Of 36 patients, (median age 10 yr) 17
had immune complex GN and 19 had pauci-immune crescentic GN. The
etiologies of the former were lupus nephritis (n=4),
postinfectious GN (3), and IgA nephropathy, Henoch Schonlein purpura and
membranoproliferative GN type II (2 each). Three patients with
pauci-immune GN showed antineutrophil cytoplasmic antibodies (ANCA).
Rapidly progressive GN was present in 33 patients, and required dialysis
in 12. At median 34 (19-72) months, 2 patients with immune complex GN
and 8 with pauci-immune GN showed renal loss. Renal survival was 94.1%
at 3 yr, and 75.3% at 8 yr in immune complex GN; in pauci-immune GN
survival was 63.2% and 54.1%, respectively (P=0.054). Risk
factors for renal loss were oliguria at presentation (hazards ratio, HR
10.50; P=0.037) and need for dialysis (HR 6.33; P=0.024);
there was inverse association with proportion of normal glomeruli (HR
0.91; P=0.042).
Conclusions: Pauci-immune GN constitutes one-half
of patients with crescentic GN at this center. Patients with pauci-immune
GN, chiefly ANCA negative, show higher risk of disease progression.
Renal loss is related to severity of initial presentation and extent of
glomerular involvement.
Key words: Antineutrophil cytoplasmic antibody, Rapidly
progressive glomerulonephritis, Vasculitis.
|
|
Crescentic glomerulonephritis (GN), characterized
by rapidly progressing renal failure, is relatively rare in childhood.
Reports are limited to case series with scant information on long-term
course [1-5]. Crescentic GN in children is commonly secondary to
postinfectious GN, Henoch Schonlein purpura and IgA nephropathy [1,3,4].
While pauci-immune crescentic GN is common in adults [6,7], there are
few reports of this condition in children [2,3].
In 1992, we described our experience on the clinical
features and renal histology in 43 consecutive patients with crescentic
GN [4]. Immunofluorescence examination showed immune complexes in 17 of
20 cases; testing for antineutrophil cytoplasmic antibodies (ANCA) was
not available. Outcomes were unsatisfactory irrespective of etiology,
with progressive renal impairment in 86% cases. During the last decade
we have noticed a reduction in proportion of patients with
postinfectious GN and increasing occurrence of pauci-immune crescentic
GN. While recent reports are limited [1,8], prompt diagnosis and
intensive therapy has resulted in better patient outcomes. The present
report describes the etiological profile and clinical course among 36
patients with crescentic GN evaluated at this centre. The short-term
course in nine patients has been reported elsewhere [9].
Methods
Hospital records were reviewed to identify children
(<18 years) diagnosed with crescentic GN between June 2001 and June 2010
and followed for at least 12-months. Records were reviewed for clinical
and biochemical features, therapies received and course. Blood was
examined for levels of creatinine, electrolytes, complement C3,
antistreptolysin O and antinuclear antibodies. Prior to 2006, ANCA were
screened by immunofluorescence; later enzyme immunoassay for
myeloperoxidase and proteinase-3 were also performed [10]. Standard
definitions were used for hematuria (>5 erythrocytes/high power field of
centrifuged specimen), proteinuria (protein to creatinine ratio, Up/Uc
³0.2 mg/mg),
nephrotic syndrome (edema, proteinuria 3-4+ or Up/Uc >2.0, and serum
albumin <2.5 g/dL) [11] and hypertension (blood pressure >95th
percentile for age, gender and height) [12].
Histology: Light microscopy examination was
considered adequate in presence of at least one core with
³10 glomeruli.
Crescents were defined as proliferation of parietal cells forming two or
more cell layers in the Bowman space; crescentic GN was the presence of
crescents in 50% or more glomeruli [6]. Specimens were examined for
cellularity of crescents, sclerosis, neutro-phil infiltration, fibrinoid
or tuft necrosis, mesangial or endocapillary hypercellularity and
tubulointerstitial changes. Immunofluorescence (IF) examination was done
for pattern and intensity of staining for immuno-globulins, C3 and C1q.
Based on staining of immune deposits and serology, histology was
classified as immune-complex GN (granular deposits of immune complexes
along capillary wall and mesangium), pauci-immune GN (scant or no
deposits, with or without positive ANCA) and anti-glomerular basement
membrane GN (linear antibody deposits). Standard criteria were used for
systemic lupus erythematosus (SLE), Henoch Schonlein purpura and
Wegener’s granulomatosis [13,14].
Therapy
Management included maintenance of fluid and
electrolyte balance, dialysis if indicated, and therapy of coexisting
infections and hypertension.
Pauci-immune GN. Remission was induced with 3-6
pulses of IV methylprednisolone (15-20 mg/kg, maximum 1 g/day), followed
by IV cyclophosphamide (500-750 mg/m 2)
for 6 doses at 3-4 week intervals, and oral prednisone (1.5 mg/kg/day
for 4 weeks, tapered to alternate-day). Patients who were dialysis
dependent also received double-volume plasma exchange for 7 days. During
the maintenance phase, patients received either azathioprine (2
mg/kg/day) or mycophenolate mofetil (500-750 mg/m2/day)
for two or more years. Additional therapies included IV immunoglobulin
and rituximab in one patient with Wegener’s granulomatosis.
Immune complex crescentic GN. Initial therapy
with IV methylprednisolone was followed by tapering doses of oral
steroids for 6 months. Patients with crescentic GN secondary to SLE, IgA
nephropathy and HSP received IV cyclophosphamide for 6 months, followed
by long-term therapy with azathioprine or mycophenolate mofetil.
Patients with postinfectious GN also received oral cyclophosphamide (2
mg/kg/day) for 12 weeks.
Monitoring and Follow up: Patients were followed
every 3-6 months. Outcome at last follow up was categorized as: (i)
complete recovery (urine protein nil/trace; serum albumin >2.5 g/dL and
estimated GFR >90 mL/min/1.73 m 2),
(ii) partial recovery (abnormal urinalysis: microscopic hematuria,
³1+
proteinuria; hypertension; or estimated GFR 60-90 mL/min/1.73 m2),
(iii) chronic kidney disease (CKD) stage 3 (GFR 30-60 mL/min/1.73
m2) and (iv) renal loss: CKD
stage 4 or 5 (GFR <30 mL/min/1.73 m2).
Statistical analysis: Data were analyzed using
STATA 11.0 (College Station, Texas). Summary statistics are presented as
median (interquartile range, IQR). Clinical features were compared using
chi square and ranksum tests; renal survival (free of renal loss) was
compared using Kaplan Meier analyses. Factors impacting outcome were
examined by Cox regression and reported as hazards ratios (HR) with 95%
confidence intervals (CI).
Results
Of 36 patients with crescentic GN, 21 were boys. The
median (IQR) age at onset of disease was 10 (8-11.5) years; 15 (41.7%)
children were younger than 10 years. The clinical and laboratory
features are presented in Table I. Based on histopathology
and IF examination, immune complex GN and pauci-immune crescentic GN
were present in 17 and 19 patients, respectively. Fifteen (88.2%)
patients with the former and 18 (94.7%) with the latter presented with
rapidly progressive GN; 3 had puffiness and mildly impaired renal
function. Six patients in each group had systemic symptoms at
presentation, including one with Wegener’s granulomatosis. Thirteen
patients with pauci-immune GN had isolated renal involvement.
TABLE I Patient Characteristics at Presentation
|
Characteristic |
Immune
|
Pauci-immune
|
P
|
|
complex |
crescentic GN
|
|
|
GN (n=17) |
(n=19) |
|
|
Boys |
11 (64.7) |
10 (52.6) |
0.46 |
|
Age, y
|
11 (10-12) |
9 (7-11) |
0.09 |
|
Duration of
|
8 (3-21) |
4 (3-16) |
0.57 |
|
symptoms, weeks |
|
Oliguria |
9 (52.9) |
12 (57.9) |
0.77 |
|
Gross hematuria |
9 (52.9) |
12 (63.2) |
0.54 |
|
Fever |
6 (35.9) |
10 (52.6) |
0.30 |
|
Seizures,
|
3 (17.7)* |
1 (5.3) |
0.24 |
|
encephalopathy |
|
Rash |
6 (35.3) |
4 (21.1) |
0.46 |
|
Arthralgia |
2 (11.8) |
2 (10.5) |
0.91 |
|
Hypertension
|
10 (58.8) |
16 (84.2) |
0.14 |
|
Creatinine, mg/dL |
1.8 (1.2-4.8) |
3.9 (1.7 - 5.3) |
0.24 |
|
Nephrotic range proteinuria |
13 (76.5) |
15 (83.3) |
0.69 |
|
Categorical variables are shown as number (percentage) and
continuous variables as median (interquartile range); *2 with
hypertensive encephalopathy; 1 with systemic lupus erythematosus. |
Histology: Adequate tissue for histopathology was
available in 35 patients; renal biopsy in one showed only 4 glomeruli, 3
of which had crescents. The median (IQR) proportion of glomeruli showing
crescents was 62% (50-89%) in patients with immune complex GN and 67%
(50-96%) in pauci-immune crescentic GN; crescents involving >80%
glomeruli were seen in 6 and 7 cases, respectively. In immune complex
GN, crescents were cellular and fibrocellular in 8 patients each (47.1%
each); one patient showed fibrous crescents. The proportion of sclerosed
and normal glomeruli was 20% (0-41%) and 14.5% (0-29%), respectively.
Tubular atrophy and interstitial fibrosis was noted in 10 biopsies,
while 8 showed chronic inflammatory infiltrate.
Biopsies in patients with pauci-immune crescentic GN
showed cellular (n=11, 57.9%), fibrocellular (n=6, 31.6%)
or fibrous (n=2, 10.5%) crescents. The median proportion of
sclerosed and normal glomeruli was 8.5% (IQR 0-40%) and 5% (0-21%)
respectively. Tubular atrophy and interstitial fibrosis were seen in 14
(73.7%) and 12 (63.2%) biopsies. Fibrin deposition was present in 12
(63.2%) cases and tuft necrosis in 4 biopsies. Faint deposits of C3 and
IgM were seen in 3 and 1 patients, respectively.
Immune complex GN was secondary to SLE in 4 patients,
and IgA nephropathy, Henoch Schonlein purpura and membranoproliferative
GN type II in two cases each; GN was postinfectious in 3 cases and
idiopathic in 5 patients. Three patients with pauci-immune
crescentic GN were ANCA positive. Two patients had microscopic angiitis
based on constitutional symptoms and specificity against myeloperoxidase
and one with chest infiltrates and specificity against proteinase-3 was
diagnosed as Wegener’s granulomatosis. Serology for ANCA was negative in
16 patients with pauci-immune GN.
Twelve (33.3%) patients required dialysis at
presentation. Eight patients, all with pauci-immune GN, underwent plasma
exchanges. Therapy for induction included IV methylprednisolone (n=31;
86.1%), IV cyclophosphamide (27; 75%) and oral cyclophos-phamide [4].
Maintenance immunosuppression included oral prednisolone (36; 100%),
azathioprine (18; 50%) and mycophenolate mofetil (11; 30.6%).
TABLE II Outcome at Last Follow Up
|
Immune
|
Pauci-immune
|
|
complex
|
crescentic |
|
GN (n=17) |
GN (n=19) |
|
Normal renal function and urinalysis
|
4 (23.5) |
3 (15.8) |
|
Abnormal urinalysis or hypertension |
7 (41.2) |
5 (26.3) |
|
Chronic kidney disease stage 2-3 |
4 (23.5) |
3 (15.8) |
|
Chronic kidney disease stage 4-5
|
2 (11.8) |
8 (42.1) |
The outcome at last follow up, at 34 (19-72) months,
is shown in Table II. Seven (19.4%) patients had
complete recovery, while 10 (30.6%) had CKD 4-5. The latter includes 3
patients who were followed for 53-121 months after renal
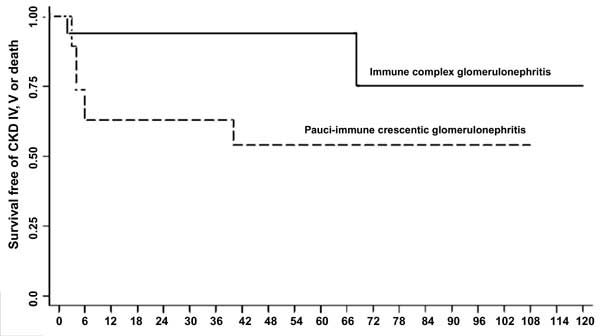
transplantation. Fig. 1 shows that renal survival was
94.1% at 1, 3 and 5-yr, and 75.3% at 8 and 10-yr in patients with immune
complex crescentic GN. Renal survival in patients with pauci-immune GN
was lower at 63.2% at 1 and 3-yr, and 54.1% at 5 and 8-yr (log rank
test; P=0.054).
 |
|
Fig. 1 Kaplan Meier survival estimates for proportion
of patients with renal loss (chronic kidney disease stage 4 and
5) or death in patients with immune complex (solid line) and
pauci-immune crescentic glomerulonephritis (interrupted line)
(log rank test P=0.054).
|
On univariate analysis, factors predicting renal loss
were presentation with oliguria (HR 10.5; 95% CI 1.16-95.25; P=0.037),
need for dialysis at presentation (HR 6.33; 1.27-31.57; P=0.024),
and the proportion of glomeruli showing fibrous or fibrocellular
crescents (HR 4.74; 0.99-22.52; P=0.050). Renal loss was
inversely correlated to the proportion of normal glomeruli (HR 0.91,
0.83-0.99; P=0.042). There was no relation between renal loss and
the presence of hypertension (P=0.22) at disease onset.
Discussion
The present report describes the etiology and outcome
of disease in children with crescentic GN evaluated at a single center
over nine years. A similar proportion of patients had pauci-immune and
immune complex GN. These findings are in contrast to previous case
series in children (Web Table I) showing that immune
complex GN constitutes the majority of cases with crescentic GN. The
causes for immune complex GN are varied and include post-infectious GN,
systemic lupus erythematosus, IgA nephropathy, Henoch Schonlein syndrome
and membranoproliferative GN [1-5, 8, 15]. Pauci-immune crescentic GN,
characterized by absence of significant glomerular immune deposits, is a
severe illness that represents an important cause of crescentic GN in
adults [6,7,16,17]. Data on pauci-immune crescentic GN is limited in
children, with most previous reports suggesting that these account for
4.6-20% of all cases [1-3,5,8]. Therefore our finding, that pauci-immune
GN constitutes more than one-half of all cases with crescentic GN,
suggests a changing etiologic profile of the illness in children.
However, the change in proportions might reflect a decline in rates of
post-infectious GN [18]. Alternatively, it may represent a referral
bias, with more cases with severe presentation, and therefore, a higher
proportion of patients with pauci-immune crescentic GN, being referred
to a tertiary care center.
A large proportion of patients with pauci-immune
crescentic GN have circulating ANCA. Since early 1990s, the application
of immunofluorescence and ELISA to detect ANCA and define its
specificities, has allowed diagnosis of ANCA-associated vasculitis,
including microscopic polyangiitis, Wegener’s granulomatosis and renal
limited vasculitis [6, 10, 14]. However, a recent review suggests that
10-30% patients with pauci-immune GN are negative for ANCA [19].
Compared to those who are ANCA positive, patients with negative serology
are younger, show less constitutional and extrarenal symptoms, have
higher proteinuria and severe lesions on histology, and an
unsatisfactory renal survival [19, 20]. A recent report from northern
India showed that 21 of 48 adults with pauci-immune rapidly progressive
GN were negative for ANCA [21]. The diagnosis of ANCA negative serology
is chiefly based on negative results on immunofluorescence [19-22].
Based on this examination, and confirmed in most by specific ELISA,
84.2% of the present patients with pauci-immune GN were negative for
ANCA. While the outcome of patients with ANCA negative pauci-immune
crescentic GN is considered unsatisfactory, we did not demonstrate
differences in view of small patient numbers.
Outcomes in crescentic GN are generally
unsatisfactory and progressive renal failure has been seen in 18.9-86% (Web
Table I) [1-5,8,15]. One-third of the present patients, followed
for median duration of three years, progressed to CKD 4-5. Compared to
our previous experience [4], the improved outcomes may reflect intensive
immunosuppressive therapy and early institution of dialysis and/or
plasmapheresis. We also found that outcome of patients with pauci-immune
GN was less satisfactory compared to immune complex GN. Of 19 patients
with the former, 8 progressed to CKD stage 4-5, compared to only 2 of 17
with the latter. The findings are similar to that in adult patients,
where pauci-immune GN has inferior outcome compared to immune complex GN
[6]. While the cellularity of crescents is a marker of outcome [1-3],
the proportion of fibrous and fibrocellular crescents was similar in
pauci-immune and immune complex GN (42.1% versus 53%) and was
unlikely to account for difference in outcomes. Other predictors of
prognosis in the present cases were similar to those reported
previously, including dialysis dependence at onset [2, 4, 6] and
proportion of normal glomeruli [23].
The current report highlights the change in
etiological profile of crescentic GN in children that mirrors trends in
adult onset disease. The distinction between subtypes based on
immunofluorescence and serological findings has important implications
for therapy and outcome. Patients with pauci-immune GN show a higher
risk of progressive kidney disease. Further studies are necessary to
characterize the natural history of disease in children with
ANCA-negative pauci-immune crescentic GN.
Contributors: AS and KP retrieved information
from case records, collated and analyzed the data. PH and AB provided
analytical inputs and guided in analysis and review of literature. AKD
reviewed and interpreted histological specimens. All authors contributed
to the preparation of the manuscript, provided significant inputs during
preparation for final publication and approved the final manuscript. AB
supervised the study and shall be its guarantor.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
• Rapidly progressing glomerulonephritis,
characterized pathologically by crescentic glomerulonephritis,
is an important cause of acute kidney injury in childhood.
• The commonest cause of crescentic
glomerulonephritis in childhood is post-infectious
glomerulonephritis, associated with deposition of immune
complexes.
What This Study Adds?
• Pauci-immune crescentic glomerulonephritis
is an important cause of crescentic glomerulonephritis in
children.
• A large proportion of patient with pauci-immune
crescentic glomerulonephritis may be negative for antineutrophil
cytoplasmic antibodies.
• The outcome of patients with pauci-immune
crescentic glomerulonephritis is unsatisfactory compared to
those with immune complex crescentic glomerulonephritis.
|
References
1. Dewan D, Gulati S, Sharma RK, Prasad N, Jain M,
Gupta A, et al. Clinical spectrum and outcome of crescentic
glomerulonephritis in children in developing countries. Pediatr Nephrol.
2008;23:389-94.
2. Jardim HM, Leake J, Risdon RA, Barratt TM, Dillon
MJ. Crescentic glomerulonephritis in children. Pediatr Nephrol.
1992;6:231-5.
3. A clinico-pathologic study of crescentic
glomerulonephritis in 50 children. A report of the Southwest Pediatric
Nephrology Study Group. Kidney Int. 1985;27:450-8.
4. Srivastava RN, Moudgil A, Bagga A, Vasudev AS,
Bhuyan UN, Sunderam KR. Crescentic glomerulonephritis in children: a
review of 43 cases. Am J Nephrol. 1992;12:155-61.
5. Tapaneya-Olarn W, Tapaneya-Olarn C, Boonpucknavig
V, Boonpucknavig S. Rapidly progressive glomerulonephritis in Thai
children. J Med Assoc Thai. 1992;75:32-7.
6. Jennette JC. Rapidly progressive crescentic
glomerulonephritis. Kidney Int. 2003;63:1164–77.
7. Koyama A, Yamagata K, Makino H, Arimura Y, wada T,
Nitta K, et al. Japan RPGN Registry Group. A nationwide survey of
rapidly progressive glomerulonephritis in Japan: etiology, prognosis and
treatment diversity. Clin Exp Nephrol. 2009;13:633-50.
8. Alsaad K, Oudah N, Al Ameer A, Fakeeh K, Al Jomaih
A, Al Sayyari A. Glomerulonephritis with crescents in children: etiology
and predictors of renal outcome. ISRN Pediatr. 2011;
doi:10.5402/2011/507298.
9. Gupta R, Singh L, Sharma A, Bagga A, Agarwal SK,
Dinda AK. Crescentic glomerulonephritis: A clinical and
histomorphological analysis of 46 cases. Ind J Pathol Microbiol.
2011;54:597-61.
10. Savige J, Pollock W, Trevisin M. What do
antineutrophil cytoplasmic antibodies (ANCA) tell us? Best Pract Res
Clin Rheumatol. 2005;19:263–76.
11. Bagga A, Mantan M. Nephrotic syndrome. Indian J
Med Res. 2005;122:13-28.
12. National High Blood Pressure Education Program
Working Group on High Blood Pressure in Children and Adolescents. The
fourth report on the diagnosis, evaluation, and treatment of high blood
pressure in children and adolescents. Pediatrics. 2004;114:555-76.
13. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ,
Rothfield NF, et al. The 1982 revised criteria for the
classification of systemic lupus erythematosus. Arthritis Rheum.
1982;25:1271-7.
14. Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K,
Davin JC, et al. EULAR/PReS endorsed consensus criteria for the
classification of childhood vasculitides. Ann Rheum Dis. 2006;65:
936–41.
15. Miller MN, Baumal R, Poucell S, Steele BT.
Incidence and prognostic importance of glomerular crescents in renal
diseases of childhood. Am J Nephrol. 1984;4:244-7.
16. López-Gómez JM, Rivera F; Spanish Registry of
Glomer-ulonephritis. Renal biopsy findings in acute renal failure in the
Cohort of patients in the Spanish registry of glomer-ulonephritis. Clin
J Am Soc Nephrol. 2008;3: 674-81.
17. Lin W, Chen M, Cui Z, Zhao M-H. The
immunopathological spectrum of crescentic glomerulonephritis: a survey
of 106 patients in a single Chinese center. Nephron Clin Pract.
2010;116:c65–c74.
18. Kanjanabuch T, Kittikowit W, Eiam-Ong S. An
update on acute postinfectious glomerulonephritis worldwide. Nat Rev
Nephrol. 2009;5:259–69.
19. Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY.
Antineutrophil cytoplasmic autoantibody–negative pauci-immune crescentic
glomerulonephritis. J Am Soc Nephrol. 2007; 18:599-605.
20. Eisenberger U, Fakhouri F, Vanhille P, Beaufils
H, Mahr A, Guillevin L, et al. ANCA-negative pauci-immune renal
vasculitis: histology and outcome. Nephrol Dial Transplant.
2005;20:1392-9.
21. Minz RW, Chhabra S, Joshi K, Rani L, Sharma N,
Sakhuja V, et al. Renal histology in pauci-immune rapidly
progressive glomerulonephritis: 8-year retrospective study. Indian J
Pathol Microbiol. 2012;55:28-32.
22. Csernok E, Ahlquist D, Ullrich S, Gross WL. A
critical evaluation of commercial immunoassays for antineutrophil
cytoplasmic antibodies directed against proteinase 3 and myeloperoxidase
in Wegener’s granulomatosis and microscopic polyangiitis. Rheumatology
(Oxford). 2002;41:1313-7.
23. Bajema IM, Hagen EC, Hermans J, Noël LH, Waldherr
R, Ferrario F, et al. Kidney biopsy as a predictor for renal
outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int.
1999;56:1751-8.
|
|
|
 |
|

