|
|
|
Indian Pediatr 2019;56: 663-668 |
 |
A Landscape Analysis of Human Milk Banks in
India
|
|
Ruchika Chugh Sachdeva 1,
Jayashree Mondkar2,
Sunita Shanbhag3,
Minu Manuhar Sinha3,
Aisha Khan3 and
Rajib Dasgupta4
From 1Maternal, Newborn, Child Health and
Nutrition, PATH, New Delhi; 2Department of Neonatology, and
3MBFI+ Project, Lokmanya Tilak Municipal Medical
College and Lokmanya Tilak Municipal General Hospital, Mumbai; and
4Department of Community Health, Jawaharlal Nehru University, New
Delhi; India.
Correspondence to: Dr Jayashree Mondkar, Professor
and Head, Department of Neonatology, Lokmanya Tilak Municipal Medical
College and Lokmanya Tilak Municipal General Hospital, Sion, Mumbai 400
022, India.
Email:
[email protected]
Received: October 26, 2018;
Initial review: January 28, 2019;
Accepted: June 20, 2019.
|
|
Objective: To evaluate the
existing status of human milk banks in India with reference to
infrastructure, human resources, funding mechanisms, operating
procedures and quality assurance. Methods: A pretested
questionnaire was administered to 16 out of 22 human milk banks across
India, operational for more than one year prior to commencing the study.
Results: 11 (69%) milk banks were in government or charitable
hospitals; only 2 (12.5%) were established with government funding. 8
(50%) had a dedicated technician and only 1(6%) had more than five
lactation counsellors. Milk was collected predominantly from mothers of
sick babies and in postnatal care wards followed by pediatric outpatient
departments, camps, satellite centers, and homes. 10 (63%) reported gaps
between donor milk demand and supply. 12 (75%) used shaker water bath
pasteurizer and cooled the milk manually without monitoring temperature,
and 4 (25%) pooled milk under the laminar airflow. 10 (63%) tracked
donor to recipient and almost all did not collect data on early
initiation, exclusive breastfeeding or human milk feeding.
Conclusion: Our study reports the gaps of milk banking practices in
India, which need to be addressed for strengthening them. Gaps include
suboptimal financial support from the government, shortage of key human
resources, processes and data gaps, and demand supply gap of donor human
milk.
Keywords: Breastfeeding, Breast
milk expression, Lactation, Storage, Prematurity.
|
|
I
ndia has the highest number of preterm births in
the world and breastfeeding rates are suboptimal [1,2]. Providing
breastmilk to these babies, especially those with very low birth weight
(VLBW), if they do not have access to their mother’s own milk due to
reasons such as mother’s sickness, separation, or temporary lactation
issues, is a challenge. This leaves them more vulnerable to infection
and death [3-5]. When mother’s milk is unavailable, pasteurized donor
human milk is recommended as the next best infant feeding option for
VLBW babies [6-8]. Human milk banks (HMBs) collect, pasteurize, test,
and store safe donor milk from lactating mothers, and provide it to
needy infants [9]. Though India’s first HMB was established in 1989,
HMBs in the country gathered momentum only in the last 3-4 years. [10].
Published data on status of these HMBs is not available. This study was
conducted to evaluate the existing status of HMBs with reference to
infrastructure, human resources (HR), funding mechanisms, operating
procedures and quality assurance in order to identify gaps to be
addressed to strengthen HMB systems.
Methods
This cross-sectional survey was conducted from August
2016 to February 2017, after obtaining permission of the Institutional
Ethics Committee at Lokmanya Tilak Municipal Medical College and General
Hospital, Mumbai. At the time of conducting the study, there were 30
HMBs in India. An online questionnaire was sent to 22 HMBs that had been
operational for more than one year at the time of start of this study.
The objective was shared telephonically with the HMB in-charge. The
questionnaire captured information on location of HMB, availability of
space, equipment and personnel, guidelines followed, operational
procedures, including donor recruitment, screening, milk collection,
processing, dispensing, utilization and infection control mechanisms,
and quality assurance measures followed during various processes,
including equipment maintenance, standard operating procedures and
hygiene protocol.
Six HMBs from different geographical zones were
visited for conducting onsite interviews of the HMB personnel to
reaffirm the data. The study team obtained informed consent of the
participants. No identifying information (names or addresses) of
respondents and facilities were retained in the electronic version of
the data records. Data were analyzed using MS Excel and primarily
included descriptive analysis, proportions and cross tabulations.
Results
Of the 22 eligible HMBs, 16 participated in the
study. Nine were run by government medical colleges, two by
charitable hospitals, and five by private hospitals. HMBs in charitable
and public hospitals were clubbed for analysis as their structure and
functioning was similar. All HMBs were operated by the Neonatology units
and were located near the neonatal intensive care units (NICU), except
one. The size of the milk processing area ranged from 100 to 862 square
feet and 200 to 300 square feet in public and private hospitals,
respectively. The setup cost of a HMB varied from INR 10,00,000 to
75,00,000. The establishment of 14 HMBs was funded by external agencies
(50% by the Rotary) and the remaining two by the government. The
recurring expenditure for ten centers were borne by the hospitals, three
were supported by the National Health Mission and two by private donors.
Availability of equipment for milk collection, storage, pasteurization
and equipment sterilization is shown in Table I. All HMBs
reported the use of electric hospital-grade breast pumps for milk
expression. In 10 HMBs, milk was stored and pasteurized in stainless
steel containers, and the remaining used polypropylene containers.
Indigenous shaker water bath was used for pasteurization in 13 HMBs, and
five used automated imported pasteurizer of which; two had both.
TABLE I Availability of Human Resources and Equipment in Public and Private Human Milk Banks (N=16)
|
Variables |
Public sector |
Private sector |
|
(n=11) |
(n=5) |
|
Human resource- Full-time staff |
|
Neonatologist/Pediatrician
|
11 (100) |
5 (100) |
|
Dedicated HMB manager |
2 (18) |
0 (0) |
|
Dedicated technician
|
7 (63.6) |
1 (20)
|
|
≥5 Lactation counselors
|
0 |
1 (20) |
|
Data entry operator |
2 (18) |
0 |
|
Human resource- Part time staff |
|
Technician |
0 (0) |
1 (20) |
|
Lactation counselors
|
0 (0) |
1 (20) |
|
Data entry operator |
1 (9) |
0 (0) |
|
Equipment |
|
Pasteurizer (shaker water bath) |
10 (91) |
3 (60) |
|
Pasteurizer (fully/semi-automatic)
|
3 (27.2) |
2 (40) |
|
Laminar air flow |
2 (18) |
2 (40) |
|
≥2 Electric breast pumps
|
11 (100) |
5 (100) |
|
>1 Deep freezer* (-20°C ±2°C) |
6 (54.5) |
3 (60) |
|
Separate deep freezers for raw and pasteurized milk |
5 (45.4) |
3(60) |
|
One freezer# |
4 (36.3) |
3(60) |
|
1 refrigerator
|
7 (63.6) |
4 (80) |
|
>1 refrigerator
|
3 (27.2) |
1 (20) |
|
All values in no.(%); #with separate shelves to
store raw milk, pasteurized milk, milk whose culture report was
awaited, and safe pasteurized milk. |
The HR available for running the HMBs are shown in
Table I. Doctors and nurses conducted counseling in three
HMBs that did not have any dedicated lactation counselors. Only two
(both private hospitals) provided breastfeeding counseling during
antenatal care. Eight HMBs had a full-time technician to undertake
pasteurization while in the rest, lactation counselors performed this
task. Milk culture in public hospitals was conducted by the hospital
laboratory services run by the Microbiology department and the private
facilities outsourced the testing. Almost all HMBs reported that they
did not collect data on early initiation, exclusive breastfeeding or
human milk feeding. Fourteen HMBs adhered to a set of guidelines, two
reported using the Human Milk Bank Association of North America (HMBANA)
guidelines [11], and one used the Perron Rotary Express Milk guidelines
(Australia) [12]. The remaining reported using their own standard
operating procedures based on HMBANA [10] or the Indian Academy of
Pediatrics guidelines [13].
Fifteen HMBs recruited donors whose babies were
admitted to the NICU and postnatal care (PNC) wards. Eight HMBS also
recruited donors from mothers visiting the well-baby clinics and the
pediatrics out-patients department (OPD). Five HMBs additionally
collected milk through community-based camps. Six HMBs also recruited
home-based donors, including one that exclusively used a home-based
model with the same mothers donating over a long period. Four HMBs had
satellite collection centers. All HMBs screened donor mothers; criteria
for acceptance included general clinical examination and negative blood
tests for HIV, Hepatitis B, and VDRL in antenatal period in the past six
months. Most HMBs pooled donor milk from multiple mothers. Two pooled at
the site of collection and four under the laminar airflow. All used the
Holder Pasteurization method. Four used fully automated pasteurizer and
the rest used shaker water bath and cooled the milk manually without
monitoring temperature. Three public HMBs conducted pre-pasteurization
culture to screen the milk. All conducted post-pasteurization cultures
and discarded milk with any positive microbiological test report. In
most HMBs, microbiological culture was done from individual containers.
Four HMBs pooled milk under the laminar flow, and conducted batch-wise
microbiological tests. Only one discarded milk as per the Bio Medical
Waste Management Guidelines
oof the hospital [14, and the rest discarded it in the sink.
Fourteen HMBs labeled the containers manually while
two labeled digitally. The labels contained information on date of
donation, batch number or container number, and date of pasteurization.
Four also mentioned expiry dates on the label. Four public and two
private HMBs stored preterm and term milk separately, and mentioned it
on the label. One private HMB also mentioned the nutritional content of
the milk. All bat one HMBs distributed pasteurized donor human milk on
prescription. Two private HMBs charged a processing fee to the
recipients. Frozen milk was transported in cold chain to the neonatology
units after which it was refrigerated and then thawed using warm water
baths before feeding. The duration between thawing and consumption was
about 2 to 6 hours in most HMBs. Ten HMBs tracked donor to recipient by
recording the donor milk batch and the container number (pool from
multiple mothers) against the recipient’s name. Only one tracked donor
milk from single mother to baby. All followed the processes outlined in
Fig. 1.
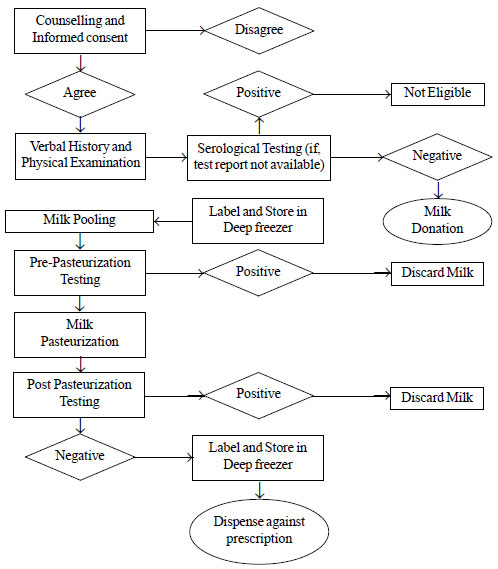 |
|
Fig. 1 Human milk bank processes in
the surveyed human milk banks.
|
All HMBs followed infection control measures. These
included donor mothers washing hands and cleaning their breasts with
water before expression. The containers in which milk was collected were
tightly secured to prevent any contamination during pasteurization.
Staff of most HMBs used disposable caps, face masks and gloves while
handling milk. The staff in all HMBs received orientation on hygiene
protocol. One HMB had a committee for infection control. The processing
room of 15 HMBs had restricted entry. 12 HMBs had equipment under Annual
Maintenance Contract (AMC); in the rest, only the pasteurizer was under
AMC. Fifteen HMBs had uninterrupted power supply.
Nearly one-fourth (range: 5%-38%) of mothers who
delivered in these hospitals donated milk. The annual number of donors,
volume of milk collected and recipient babies is presented in
Table II. Informed consent for donation was obtained in 13
facilities, and consent from recipient’s parents/guardians was obtained
in 14 facilities. Most provided donated milk to preterm babies,
sick full-term neonates in NICU, orphans, or babies of mothers with poor
lactation. Facilities reported 30% to 50% of the babies in NICU and 10%
to 20% babies in PNC ward required DHM for a variable period after
birth. Ten HMBs reported gaps between demand and availability of DHM.
Processed donor milk was used within a week or fortnight because of high
demand in most HMBs. DHM was fed to VLBW babies for 5-15 days on an
average during hospital stay.
TABLE II Annual Statistics of Donors and Recipients in Participating Human Milk Banks (N=16), 2015-16
|
Variables |
Median (Range) |
Public sector*
|
Private sector* |
|
Number of donors
|
600 (70-4000) |
1938 |
316 |
|
Volume of milk collected (liters) |
382 (30-1085) |
498 |
230 |
|
Volume of banked milk utilized (liters) |
293 (27-1047) |
469 |
174 |
|
Number of recipients
|
500 (80-3993) |
1480 |
261 |
|
*Average number at the participating centers. |
Discussion
This study offers an insight into the state of HMBs
in our country and serves as a baseline assessment of HMBs. A limitation
of this study was that onsite visits to all HMBs were not carried out
for confirmation of these findings. Moreover, about one-fourth of the
eligible HMBs did not participate in the survey, and few questions were
left unanswered by participating centers. Analysis was performed based
on the information shared by the facilities, and is prove to ‘reporting
bias’ .
We noted wide variations in the cost of establishing
HMBs across facilities. Imported automated pasteurizer and hospital
grade breast pumps accounted for the bulk of the capital costs.
Innovations are needed for developing indigenous and cost effective
models for these. equipments. Most HMBs were established with funding
from external agencies suggesting the need for a greater government
involvement for establishing and running HMBs, The Brazilian Network of
human milk banking has successfully demonstrated the effectiveness of a
government supported, nationalized, human milk banking as part of
integrated breastfeeding program [9]. There is a need to have more
lactation counselors and dedicated technicians in facilities to ensure
availability of quality lactation support to mothers, feeding data, and
safe DHM. Need for continuous support to mothers for breastfeeding has
been reiterated globally which in turn motivates mothers to donate milk
[15,16]. Our study analyzed that DHM can benefit five million babies
annually in India. Donor milk was collected from multiple sites in the
participating centers. However, community- and satellite centre-based
collection need to be encouraged to close the demand-supply gap as many
HMBs reported having short supply. Standardized guidelines on collection
of milk through camp and home based donors are needed.
More than half of the HMBs used stainless steel
containers for collection, which is unique to India. Steel has good
conductivity which may help in faster heating and cooling cycles and
should preserve milk nutrients better. However, such data comparing the
effect of steel, glass and polypropylene containers on the composition
of the stored milk are not available. The use of laminar airflow in more
HMBs will ensure aseptic pooling of milk. Pre-pasteurization culture of
milk, which is not being followed across all HMBs, should be implemented
uniformly.
The National Guidelines on Lactation Management
Centers in Public Health Facilities released by the Government of India
in 2017 positions HMBs as comprehensive lactation management centers
(CLMC) to universalize access to breast milk for babies. CLMCs support
breastfeeding and milk expression for sick babies, encourage kangaroo
mother care and provide pasteurized donor human milk to needy babies
lacking access to mother’s milk [17]. The gaps brought out by this study
should be addressed during the scaling up of HMBs/CLMCs as per the
national guidelines. Next steps in scaling up involve ensuring
sustainable funding and human resources, technology innovation, ensuring
uniformity of quality assurance procedures and standards including
operationalizing Hazard Analysis Critical Control Points systems,
accreditation, robust data tracking system and recording feeding data
for informed program decision making. Given the suboptimal status of
newborn nutrition in India, strengthening HMBs will contribute to
increasing access to lifesaving human milk for all babies, as part of
newborn nutrition and care.
Acknowledgments: Dr S Sitaraman, SMS Medical
College, Jaipur; Dr Poonam Singh, SMIMER, Surat; Dr Suchandra Mukherjee,
Institute of Post-Graduate Medical Education and Research and Seth
Sukhlal Karnani Memorial Hospital, Kolkata; Dr Kumutha, Government
Hospital for women and children, Chennai; Dr Kamalrathnam, Institute of
Child Health, Chennai; Dr. Sandhya S Khadse, BJ Government Medical
college and Sassoon General Hospital, Pune; Dr Shailaja Mane, Dr DY
Patil Medical College, Hospital and Research Centre, Pune; Dr Umesh
Vaidya, Sahyadri Hospital, Pune; Dr Rajan Joshi, Deenanath Mangeshkar
Hospital and Research Centre, Pune; Dr Vandana Kumavat, Rajiv Gandhi
medical college, Chhatrapati Shivaji Maharaj Hospital, Thane; Dr Swati
Manerkar, LTMMC and GH, Mumbai; Dr Ruchi Nanavati, KEM Hospital, Mumbai;
Dr Sudha Rao, Wadia Hospital , Mumbai; Dr Rajesh Boob, Dr PDM Medical
College, Amaravati; Dr Jaishree Kulkurni; Fernandez Hospital, Hyderabad;
and Dr Raghuram Mallaiah; Fortis La Femme, New Delhi for their support
in facilitating data collection. Our special gratitude and
acknowledgment is extended to Kiersten Israel-Ballard Associate
Director, Maternal, Newborn and Child Health and Nutrition Global
Program, PATH and Sudip Mahapatra, Regional Monitoring and Evaluation
Specialist, PATH, for reviewing the research design and report. We also
extend our thanks to Paramita Kundu for her support in writing this
manuscript and Manu Bhatia for providing editorial support.
Contributors: RCS: conceptualized the research
design and protocol, provided inputs on the research tool, analysis and
interpretation of the data, and contribute to manuscript writing and
revised it; JM: administrative and technical guidance on the research,
design of research, and provided critical inputs on writing of the
manuscript; SS: contributed to research design, conceptualized the
research tool, and inputs to manuscript writing; MMS and AK: data
collection, analysis, manuscript writing; RD: analyzed the study
results, supported interpretation and contributed to the manuscript
writing and revision. All authors approved the final version of
manuscript, and are willing to be accountable for all aspects of study.
Funding: This project was funded through a grant
from the Margaret A. Cargill Philanthropies to PATH.
Competing Interests: None stated.
|
What This Study Adds?
•
The survey identified few gaps
in human milk banks in India that need to be addressed to
strengthen these facilities for better neonatal outcomes.
|
References
1. March of Dimes, PMNCH, Save the Children, WHO.
Born Too Soon: The Global Action Report on Preterm Birth. Eds Howson CP,
Kinney MV, Lawn JE. Geneva: World Health Organization, 2012.
2. International Institute for Population Sciences
(IIPS) and ICF. National Family Health Survey (NFHS-4), 2015-16: India.
Mumbai: IIPS; 2017.
3. Panczuk J, Unger S, O’Connor D, Lee SK. Human
donor milk for the vulnerable infant: A Canadian perspective. Int
Breastfeed J. 2014;9:4.
4. Cregan MD, De Mello TR, Kershaw D, McDougall K,
Hartmann PE. Initiation of lactation in women after preterm delivery.
Acta Obstet Gynecol Scand. 2002; 81:870-7.
5. PATH. Policy Brief: Ensuring Equitable Access to
Human Milk for all Infants: A Comprehensive Approach to Essential
Newborn Care. Seattle: PATH; 2017. Global Breastfeeding Collective.
Available from: https://path. azureedge.net/media/documents/MNCHN_Equitable
AccesstoHumanMilk_PolicyBrief.pdf. Accessed November 19, 2017.
6. WHO. Every Newborn: An Action Plan to End
Preventable Deaths. Geneva: World Health Organization, 2014. Available
from:https://apps.who.int/iris/bitstream/handle/10665/127938/9789241507448_eng.pdf;jsessionid
=C3B 6F4BB283F8EA1A4606F4EE228CB21?sequence=1. Accessed January 8,
2018
7. Committee on Nutrition, Section on Breastfeeding,
Committee on Fetus and Newborn. Donor human milk for the high-risk
infant: Preparation, safety, and usage options in the United States.
Pediatrics. 2017;139:e20163440.
8. ESPGHAN Committee on Nutrition, Arslanoglu S,
Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al.
Donor human milk for preterm infants: Current evidence and research
directions. J Pediatr Gastroenterol Nutr. 2013;57:535-42.
9. DeMarchis A, Israel-Ballard K, Mansen KA, Engmann
C. Establishing an integrated human milk banking approach to strengthen
newborn care. J Perinatol. 2017;37:469-74.
10. Nangia S, Chugh RS, Sabharwal V. Human milk
banking: An Indian experience. Neoreviews. 2018;19:e201.
11. Human Milk Banking Association of North America.
Guidelines for the Establishment and Operation of a Donor Human Milk
Bank; 2015.
12. Hartmann BT, Pang WW, Keil AD, Hartmann PE,
Simmer K; Australian Neonatal Clinical Care Unit. Best practice
guidelines for the operation of a donor human milk bank in an Australian
NICU. Early Human Dev. 2007;83: 667-73.
13. Bharadva K, Tiwari S, Mishra S, Mukhopadhyay K,
Yadav B, Agarwal RK, et al. Human Milk Banking Guidelines Indian
Academy of Pediatrics. Indian Pediatr. 2014;51:469-74.
14. Government of India Ministry of Environment,
Forest and Climate change. Available from:http://www.moef.nic.in/sites/default/files/Final_vetted_BMW%20
Rules%202015. pdf. Accessed November 16, 2017.
15. Leung JCY, Yau SY. Perceptions of breastfeeding
mothers on breast milk donation and establishment of human breast milk
bank in Hong Kong: A Qualitative Study. International J Nursing.
2015;2:72-80.
16. Debortoli de MW, Christina PM, Fatima FMI,Palmira
FB. Representations of women milk donors on donations for the human milk
bank. Cadernos Saúde Coletiva. 2016;24:139-44.
17. Child Health Division, Ministry of Health and
Family Welfare, Government of India. National Guidelines on Lactation
Management Centres in Public Health Facilities. Available from:
http://nhm.gov.in/images/pdf/programmes/IYCF/National_Guidelines_Lactation_
Management_Centres.pdf. Accessed November 16, 2017.
|
|
|
 |
|

