|
|
|
Indian Pediatr 2016;53: S57-S60 |
 |
Role of Global Alliance
for Vaccines and Immunization (GAVI) in Accelerating Inactivated
Polio Vaccine Introduction
|
|
Naveen Thacker, #Deep
Thacker and #Ashish
Pathak
From Deep Children Hospital and Research Centre,
Gandhidham, and #Department of Pediatrics, RD Gardi Medical
College, Ujjain, Madhya Pradesh, India.
Correspondence to: Dr Ashish Pathak, Professor,
Department of Pediatrics, RD Gardi Medical College, Ujjain, Madhya
Pradesh, India.
Email:
[email protected]
|
Global Alliance for Vaccines and Immunization (GAVI, the Vaccine
Alliance) is an international organization built through public-private
partnership. GAVI has supported more than 200 vaccine introductions in
the last 5 years by financing major proportion of costs of vaccine to 73
low-income countries using a co-financing model. GAVI has worked in
close co-ordination with Global Polio Eradication Initiative (GPEI)
since 2013, to strengthen health systems in countries so as to
accelerate introduction of inactivated polio vaccine (IPV). GAVI is
involved in many IPV related issues like demand generation, supply,
market shaping, communications, country readiness etc. Most of the 73
GAVI eligible countries are also high priority countries for GPEI. GAVI
support has helped India to accelerate introduction of IPV in all its
states. However, GAVI faces challenges in IPV supply-related issues in
the near future. It also needs to play a key role in global polio legacy
planning and implementation.
Keywords: GAVI, the Vaccine Alliance, Global Polio
Eradication Initiative, IPV.
|
|
G
lobal Alliance for Vaccines and Immunization
(GAVI, the Vaccine Alliance) is an international organization, which was
created as a public-private partnership. GAVI brings together United
Nations Childrenís Fund (UNICEF), the World Bank, the vaccine
manufactures from resource rich and resource poor countries, donors from
the resource rich countries, and representatives from governments of the
low-income countries across the world and Civil Society Organizations
(CSOs)[1]. Since its inception in 2000, GAVIís support has contributed
to the immunization of an additional 500 million children in low-income
countries and has averted 7 million deaths due to vaccine preventable
diseases. GAVI has supported more than 200 vaccine introductions and
campaigns in low-income countries during the 2011-2015 period [1].
The mission of GAVI is "Saving childrenís lives and
protecting peopleís health by increasing equitable use of vaccines in
lower-income countries" [1]. GAVI is dependent on the effectiveness of
the countries health-system to deliver life-saving vaccines, thus GAVI
supports countries to strengthen country health system by proving health
system strengthening (HSS) grants. Both GAVI and GPEI have committed to
strengthen immunization programs to introduce IPV and withdraw oral
polio vaccine (OPV) as per the Polio Eradication Endgame Strategic Plan
2013-2018 [2]. The GAVI board and GPEI recognized the synergies to work
together and developed common goals, objectives, oversight mechanisms,
accountability mechanism and common program management as shown in
Fig. 1.
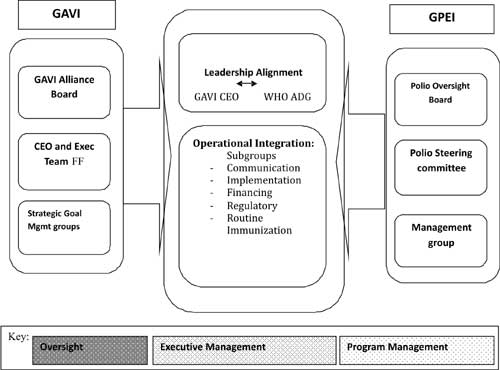 |
|
Fig.1 *GAVIís framework for
co-ordination with Global Polio Eradication Initiative.
*Adopted from Document 11a-GAVIís complimentary role on
Polio-approach available from
http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_CH6_EN_US.pdf.
|
Basis of GAVI support to Countries. GAVI
invites applications for support from governments of those countries
whose gross national income per capita is below GAVIís eligibility
threshold, this threshold was US dollar 1580 in the year 2015 [3]. Based
on the eligibility threshold, 73 countries are eligible for GAVI
support. GAVI purchases vaccines through UNICEF, and provides them to
governments whose applications are approved [3,4].
GAVI Alliance Complements Polio Eradication Efforts
The overall objective of GAVIís engagement with polio
eradication is a complimentary approach to the GPEI, which is "to
improve immunization services in accordance with GAVIís mission and
goals while supporting polio eradication by harnessing the complementary
strengths of GAVI and GPEI in support of countries" [5].
The most important issues related to IPV introduction
that GAVI is helping to resolve are: (a) demand, supply and
market-shaping implications; (b) communications and country
dialogue on IPV introduction; (c) IPV implementation including
prioritisation of countries, countryís readiness, and preparation for
introduction, and (d) initial projections of financial resource
requirements [5].
GAVIís support to countries ensures that the
countries with stretched and burdened healthcare and immunization
systems receive technical assistance. To achieve this GAVI works with
country partners WHO, UNICEF and CSOís to develop training material,
traine the healthcare workers to overcome communication challenges,
develop immunization-tracking system, and strengthen cold chain and cold
chain management capacity. GAVI has also encouraged countries to look at
IPV introduction in a larger global context of polio endgame strategy.
GAVI has ensured that material and tools for the best practice for
administration of multiple vaccinations are also provided to the
countries with the help of UNICEF country offices.
For the introduction of IPV, GPEI has prioritized
countries in four tiers, based on three criteria; endemicity of wild
poliovirus, history of cVDPV emergence, and routine immunization
coverage (Table I) [6]. Tier 1 contain the highest
priority countries for IPV introduction and include the endemic
countries. All tier 1 countries except China are GAVI countries. Most
tier 2 countries are also GAVI eligible countries. The concentration of
GAVI countries in tiers 1 and 2 affirms the importance of GAVI policies,
which incentivize rapid introduction [6].
TABLE I Priority of Countries for IPV Introduction
|
Tier |
Description of criteria |
Number of countries
|
% of OPV birth control
|
|
Tier1 |
WPV endemic countries OR countries that have reported a cVDPV2
since 2011 |
14 |
61% (38% attributable to India and China.)
|
|
Tier 2 |
Countries who have reported a cVDPV1/cVDPV3 since 2001 OR
large/medium2 sized countries with DTP3 coverage <80% in
2009,2010, 2011 as per WUNIC
|
19 |
11%
|
|
Tier 3 |
Large/ medium2 countries adjacent to Tier 1 countries that
reported |
14 |
11%
|
|
WPV since 2003 OR countries that have experience a WPV
|
|
|
|
importation since 2011
|
|
|
|
Tier 4
|
All other OPV only using countries
|
77 |
17% |
IPV Introduction in India
India has always been special for GAVI because with a
birth-cohort of almost 27 million, India is the most populous
GAVI-eligible country [7]. Also, India still accounts for one-fifth of
child deaths worldwide and more than a quarter of all under-immunised
children in GAVI-eligible countries [7]. India remains eligible for GAVI
support based on its GNI level [7]. Yet, given the large birth cohort of
the country, GAVI has limited its support to catalytic funding to India.
Until 2011, there was a limit placed on GAVI support to India, which was
removed with the condition that the Board continues to review any new
support case-by-case [7].
The National Technical Advisory Group on Immunization
(NTAGI), the apex body for decision-making on immunization related
issues in India, recommended a comprehensive IPV introduction plan to
the Government of India (GoI) [8]. India applied for funding to GAVI in
September 2014, for the period of September 2015 to 2018 at an estimated
cost of US dollar 160 million. In November 2015, India launched IPV in
six states and has recently expanded it to all states and Union
Territories [9].
Health System Strengthening (HSS) Support to India
GAVI has disbursed US dollar 30.6 million for the IPV
introduction and US dollar 107 million in the year 2014-15 [10]. The
grant has been focused by GoI for use in 12 states and 127
underperforming districts and is synergistic to Mission Indradhanush
[10]. Specifically, the grant has been used to strengthen the cold chain
management. To enhance human resource capacity, National cold chain
vaccine management resource center has been established in New Delhi
[10]. National cold chain training center has been strengthened in Pune
[10]. In 20 districts across UP, MP and Rajasthan, electronic vaccine
intelligence network (eVIN) has been implemented to enable real time
information on cold chain temperatures, vaccine stocks and flows [10].
To increase demand for routine vaccination, National behavioral change
and communication (BCC) strategy has been developed and immunization
messages have been developed and broadcast through mass media [10]. The
National monitoring and evaluation plan for immunization has been
drafted and monitoring and evaluation of routine immunization is
currently functional in 24 of the 36 states across India [10]. Two
rounds of survey for National Immunization Coverage Evaluation have been
done in 2015. This evaluation will further identify low performing
districts for routine immunization coverage [10]. Guidelines for tagging
high-risk low coverage areas have been developed along with WHO India
National polio surveillance program (NPSP) [10].
Financing the Polio End Game Globally
Globally, US dollar 11 billion have been invested in
the GPIE since its inception in 1988 [2]. A total of US dollar 5.5
billion are being invested for the polio eradication and endgame
strategic plan [2]. An investment of US dollar 5.5 billion today in
polio eradication is expected to yield up-to US dollar 40 to 50 billion
in additional net benefit in subsequent 20 years for the worldís poorest
countries [11]. The bases of calculation for these gains are from
avoided treatment costs and productivity gains [11]. Today, more than 10
million people are walking who would otherwise have been paralyzed by
the poliovirus [12].
Challenges for the Future
There have been challenges in the availability of IPV
because of which 28 countries had to delay their planned dates to
introduce IPV. In eight countries, the IPV introduction was delayed to
after the trivalent to bivalent OPV switch, after April 2016. It is
expected that supply constraints will remain till end of 2018 because of
two main reasons: firstly, delays in the manufacture production scale-up
due to technical reasons; and secondly, increased use of IPV in
campaigns [9].
Globally there is a need to plan for the "polio
legacy". The term "polio legacy" refers to the investments made
in polio eradication that can be shifted to meet other crucial health
goals [13]. Strengthening health systems to increase coverage levels in
routine immunization to more than 85% for DPT3 in all districts across
countries globally, including India, is one of the most important
challenges. Strengthening systems to introduce IPV will also catalyze
the delivery of other lifesaving vaccines like pneumococcal, rotavirus
and human papilloma virus which are in line to be introduced in National
immunization schedule by GAVI support [7]. One practical programmatic
problem, which India avoided, was shortage of cold chain space as it
introduced IPV in states where pentavalent vaccines were already
introduced. From scientific point of view, we need more research into
economic aspects of the vaccination program including impact of new
vaccine introduction, cost of delivering a vaccine in rural, urban and
tribal areas, methods to evaluate vaccine effectiveness and decision
tools for policy makers. From a practicing pediatrician point of view we
need to ensure that a child that is eligible to receive polio should not
leave a clinic without receiving a polio vaccine.
Funding: None; Competing interest: NT is
GAVI Board member representing civil society organizations and AP is
special advisor to NT.
References
1. GAVI 2016. Every Child Counts: The Vaccine
Alliance Progress Report 2014. Available from
http://www.GAVI.org/progress-report/. Accessed May 13, 2016.
2. GPEI 2013. Polio Eradication and Endgame Strategic
Plan 2013Ė2018. Available from
www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/EndGameStratPlan_20130414_ENG.pdf.
Accessed May 13, 2016.
3. Kallenberg J, Mok W, Newman R, Nguyen A, Ryckman
T, Saxenian H, et al. GAVIís Transition Policy: Moving From
Development Assistance To Domestic Financing Of Immunization Programs.
Health Affairs. 2016;35:250-8.
4. Shen AK, Weiss JM, Andrus JK, Pecenka C, Atherly
D, Taylor K, et al. Country ownership and GAVI transition:
Comprehensive approaches to supporting new vaccine introduction. Health
Affairs. 2016;35:272-6.
5. GAVI Alliance Polio Eradication Efforts, Available
from
http://www.GAVI.org/Library/News/GAVI-features/2014/GAVI-Alliance-complements-global-polio-eradication-effort/.
Accessed May 13, 2016.
6. WHO-Risk tiers for IPV introduction, Available
from http://www.who.int/immunization_standards/vaccine_
quality/4a_risk_tiers_for_ipv_introduction.pdf. Accessed May 13,
2016.
7. GAVI 2015. Alliance Partnership Strategy with
India, 2016-2021 Report to the Board 3rd December 2015. Available from
http://www.GAVI.org/Library/News/Press-releases/2016/Historic-partnership-between-GAVI-and-India-to-save-millions-of-lives/.Accessed
May 13, 2016.
8. WHO 2016 India Expert Advisory
Group (IEAG) February 26, 2016 meeting. Available from
http://www.who.int/immunization/sage/meetings/2016/april/3_Conclusions_
recommendations_mini_IEAG_26_Feb2016_NewDelhi.pdf. Accessed May 12,
2016.
9. Report from GAVI to at SAGE on Immunization
October 2015. Available from
http://www.who.int/immunization/sage/meetings/2015/october/Berkely_GAVI_SAGE_
October2015.pdf. Accessed May 13, 2016.
10. Joint Appraisal Report India 2015, Available from
http://www.GAVI.org/country/india/documents/#approve dproposal.
Accessed May 13, 2016.
11. Duintjer Tebbens RJ, Pallansch MA, Cochi SL,
Wassilak SGF, Linkins J, Sutter RW, et. al. Economic analysis of
the global polio eradication initiative. Vaccine 2011;29: 334-43.
12. Direct benefits of eradication and risks of polio
reintroduction. Available from www.polioeradication.org
http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_CH4_EN_US.pdf.
Accessed May 13, 2016.
13. Cochi SL, Hegg L, Kaur A, Pandak C, Jafari H. The
global polio eradication initiative: Progress, lessons learned, and
polio legacy transition planning. Health Aff. 2016;35:277-83.
|
|
|
 |
|

