|
|
|
Indian Pediatr 2021;58:853-856 |
 |
Outcome of Neonates Born to COVID-Positive
Women at 6 Months of Age
|
|
Dinesh Munian,
1
Rituparna Das,1
Avijit Hazra,2
Somosri Ray1
From Department of 1Neonatology, Medical College and
Hospital, Kolkata, West Bengal; 2Department of Pharmacology,
Institute of Postgraduate Medical Education & Research (IPGME&R) and
SSKM Hospital, Kolkata, West Bengal.
Correspondence to: Dr Somosri Ray, Department of Neonatology, Medical
College and Hospital,
Kolkata 700 073, West Bengal.
Email: [email protected]
Received: March 13, 2021;
Initial review: April 15, 2021;
Accepted: July 09, 2021.
Published online: July 23, 2021;
PII:S097475591600354
|
Objective: To compare clinical and
neurodevelopmental outcome at the age of 6 months for neonates born to
SARS-CoV-2-positive mothers. Methods: Neonates of SARS-CoV-2
positive mothers, admitted in our hospital were assessed for growth,
neurodevelopment by Amiel-Tison method, and Developmental Profile (DP3)
at discharge as part of another study (July 2020). This data were
retrieved and babies followed-up at the age of 6 months. Composite
adverse outcome was death within 6 months post discharge or DP3 score
<70 and hearing/visual deficit. Results: Out of 131 enrolled at
discharge, 127 (97%) were followed up. SARs-CoV-2 positive neonates
(Group I; 19, 15%) had more symptoms (P=0.012), sepsis (P=0.014),
pneumonia (P=0.029), longer hospital stay (P<0.001)
following birth compared to group II (SARs-CoV-2 negative neonates;108,
85%). No baby in group I met definition of composite adverse outcome,
while in group II it was 0.9% (1 child with DP3 <70 with hearing
deficit) (P=1.0) without any difference in hospital readmission,
growth, DP3 scores, or tone abnormalities. Conclusions: There is
no difference in growth, neurodevelopment, and hospital readmission in
early infancy among infected and non-infected babies born to SARS-CoV-2
positive mothers.
Keywords: Corona virus, SARs-CoV-2, Neonate,
Neurodevelopment.
|
|
A
dverse pregnancy
outcomes have been
documented with two earlier pathogenic
coronavirus infections severe acute
respiratory syndrome (SARS) and Middle East respiratory syndrome
(MERS) [1]. However,
most severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) -positive neonates (50%) were symptomatic with
predominant respiratory symptoms attributed to coronavirus
disease (COVID) [2,3] and required intensive care. Among
symptomatic SARS-CoV-2 positive neonates, morbidities also
relate to prematurity and perinatal events [4].
Information on long term outcome of neonates
following COVID-19 is lacking so far. Only a handful of studies
are available following SARS
[5,6]. Hence, we planned to assess clinical and
neurodevelopmental outcome in early infancy for neonates born to
SARS-CoV-2 positive mothers.
METHODS
After institutional ethics committee
approval, information was retrieved from hospital records for
the present study. Demographic details, clinical features,
hospital course, and SARS-CoV-2 positively status were collected
for all neonates born to SARS-CoV-2 positive mothers during May
to July, 2020, as part of a previous study [unpublished data].
Nasopharyngeal and oropharyngeal swabs for
COVID-19 real time-polymerase chain reaction (RT-PCR) were sent
at 24-48 hours of life [7]. For outborns, if admitted beyond 48
hours, RT-PCR test was done at admission. The test was repeated
immediately, if new symptoms appeared, even if the first test
was negative; otherwise test was repeated after 5 days. For
SARS-CoV-2 positive neonates, repeat test was done after 10 days
and they were discharged, if negative.
After parental consent, the children were
assessed in the neonatal follow-up clinic at 14 days following
discharge, then at 6 weeks, 3 months and 6 months of corrected
age. Weight, length and head circumference were measured using
electronic weighing scale, infantometer, non-stretchable
fiberglass tape, respectively and plotted on WHO growth chart
[8]. The advanced or delayed development across five domain
scores physical, adaptive behavior, social-emotional,
cognitive and communication and the general development score
were plotted at 6 months of corrected age as per Developmental
Profile 3 (DP3) manual by a single investigator [9]. Children
were classified as per following scheme: Score <70 delayed,
70-84 below average, 85-114 average, 115-130 above
average, and >130 well above average.
Neurological examination was done by a single
investigator as per Amiel-Tison method [10]. Retinopathy of
prematurity (ROP) screen, if indicated, and brainstem evoked
response audiometry (BERA) with age-appropriate behavioral
audiometry were done at follow-up. During follow-up, parents
were interviewed with pre-tested and pre-validated questionnaire
containing questions on details of their babys readmission (if
any till date). The details of readmission were confirmed by
checking the discharge certificates or verified from medical
records if readmitted in our hospital. All babies readmitted in
our hospital underwent RT-PCR for SARS-CoV-2.
Primary outcome was adverse composite outcome
defined as death within 6 months post discharge or developmental
delay (defined as DP3 score <70) with hearing/visual deficit.
Secondary outcomes were DP3 scores, hearing, visual deficit,
abnormal tone, growth z-scores at follow-up, hospital
readmission rate, noninvasive/invasive respiratory support days
during readmission.
Statistical analysis: Numerical
variables were compared between groups by Student independent
samples t test, if normally distributed or by
Mann-Whitney U test, if otherwise. Fisher exact test or
Pearson chi-square was employed for intergroup comparison of
categorical variables. All analyses were two-tailed and
statistical significance was set at P < 0.05 for all
comparisons.
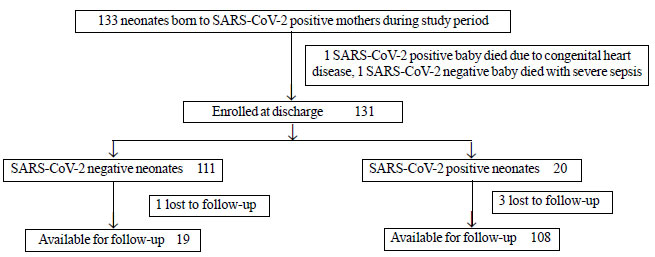
RESULT
Out of 131 enrolled neonates, results of 127
(97%) babies were analyzed (Fig. 1). All mothers
were RT-PCR positive at median (IQR) of 5 [2,8] days before
delivery. All symptomatic SARS-CoV-2 positive neonates (n=10)
had sepsis like manifestations (Table I). None had
meconium aspiration syndrome, hyaline membrane disease or
moderate to severe perinatal asphyxia. SARS-CoV-2 positive
neonates (group I) were more symptomatic (P=0.012), more
commonly had sepsis (P=0.014) or pneumonia (P=0.029),
and had longer duration of hospital stay (P<0.001)
compared to group II.
 |
|
Fig. 1 Study flow chart.
|
Table I Demographic and Clinical Details of Neonates Born to SARS-CoV-2 Positive Mothers (N=127)
| Parameters |
SARS-CoV-2 |
SARS-CoV-2 |
|
positive |
negative |
|
(n = 19) |
(n = 108) |
| Gestational age (wk)a |
37 (36, 38) |
37 (36, 38) |
| Birthweight (g)a |
2765 |
2700 |
|
(2300, 3135) |
(2231, 3000) |
| Male sex |
12 (63.1) |
62 (57.4) |
| Small for gestational age |
4 (21) |
28 (25.9) |
| Vaginal delivery |
14 (73.7) |
66 (61.1) |
| Age at RT-PCR sampling (h)a |
48 (38, 96) |
48 (40, 79) |
|
Hospital stay after birth (d)a,b |
10 (8, 16) |
6 (3, 7) |
|
Symptomatic babiesc |
10 (52.6) |
23 (21.3) |
| Respiratory distress |
7 (36.8) |
21 (19.4) |
|
Transient tachypnea of newborn |
1 (5.2) |
6 (5.5) |
| Pneumoniad |
4 (21) |
5 (4.6) |
| Poor feeding/lethargy |
3 (15.7) |
7 (6.5) |
| Vomiting |
2 (10.5) |
7 (6.5) |
| Diarrhea |
2 (10.5) |
3 (2.7) |
| Hypothermia |
1 (5.3) |
2 (1.8) |
| Shock |
1 (5.2) |
1 (0.9) |
| Seizure |
2 (10.5) |
2 (1.8) |
|
Probable sepsisc,e |
9 (47.4) |
19 (17.6) |
| Culture positive sepsis |
1 (5.3) |
3 (2.7) |
| Meningitis |
1 (5.3) |
2 (1.8) |
| Duration of antibiotics (d)a |
6 (5, 14) |
7 (5, 8.5) |
| Duration of oxygen (h)a |
48 (39, 84) |
42 (24, 48) |
|
Data in no. (%) or
aMedian (IQR). RT-PCR: Real time polymerase chain
reaction, TTNB: Transient tachypnea of newborn. bP<0.001,
cP=0.001. dP=0.03. eSepsis screen positive culture
negative sepsis accounted for probable sepsis.
SARS-CoV-2: Severe acute respiratory syndrome
coronavirus 2. Positive/negative as per real time
polymerase chain reaction (RT-PCR). |
There was no death post-discharge. During
follow-up, no infant in group I met definition of composite
adverse outcome, while in group II one child (0.9%) had DP3
score <70 with hearing deficit (P=1.0). There were no
differences in DP3 scores and anthropometry among the two groups
(Table II). BERA was done in 2 out of 19 babies in group
I, which was normal in all, while in group II, it was done in 10
babies and was normal in 9 babies (P=1.0). No baby had
abnormal ROP (done for only 9 babies). No babies other than one
with delayed develop-ment in group II had abnormal tone.
Table II Follow-up Data at 6 Months for Neonates Born to SARS-CoV-2 Positive Mothers
| Parameters |
SARS-CoV-2 |
SARS-CoV-2 |
|
positive |
negative |
|
(n = 19) |
(n = 108) |
| Weight (g)a |
6850 (893) |
6820 (754) |
| < -3 z-score |
2 |
10 |
| -3 to -2 z score |
3 |
9 |
| -2 to 0 z score |
10 |
75 |
| 0 to +2 z score |
3 |
12 |
| +2 to +3 z score |
1 |
2 |
| Length (cm)a |
64.8 (3.1) |
64.9 (2.6) |
| < -3z score |
2 |
10 |
| -3to -2 z score |
4 |
9 |
| -2 to 0 z score |
9 |
75 |
| 0 to +2 z score |
3 |
11 |
| +2 to +3 score |
1 |
3 |
| Head circumference (cm)a |
41.6 (1.6) |
41.5 (1.4) |
| < -3z score |
1 |
4 |
| -3 to -2 z score |
3 |
11 |
| -2 to -1 z score |
3 |
52 |
| -1 to 0 z score |
8 |
27 |
| 0 to +1 z score |
4 |
14 |
| Development assessment |
|
|
|
General developmental scorec
|
87.4 (12.3) |
90.6 (10.1) |
|
Developmental categoryc |
|
|
| Below average |
8 (42.1) |
28 (25.9) |
| Delay |
1 (5.2) |
1 (0.9) |
| Readmission related |
|
|
|
Babies readmittedc |
2 (10) |
7 (6.5) |
| Age at readmission (d)a |
105 (21) |
68 (45) |
| Duration of antibiotic (d)a |
3 (0) |
5.3 (1.5) |
| Duration of oxygen (d)
(n=7)b |
3 (0, 0) |
4 (3, 4) |
|
DP3:Developmental
profile 3, BERA: Brainstem evoked response
audiometry, ROP: Retinopathy of prematurity. a Mean
(SD), b Median (IQR), c n (%). All P>0.05. |
Seven babies from group II (pneumonia 3,
bronchiolitis 1, viral upper respiratory infection and diarrhea
1, sepsis with poor feeding and lethargy 2) and two from group I
(bronchiolitis 1, diarrhea 1) were readmitted. SARS-CoV-2 RT-PCR
were negative in all 9 readmitted babies. None required
noninvasive or invasive mode of ventilation following
readmission. There was no difference in course on readmission (Table
II).
DISCUSSION
In our study, no SARS-CoV-2 positive neonate
in infancy met definition of Composite adverse outcome, at 6
months while it was 0.9% in the other group.
Neonates are said to be exposed to SARS-CoV-2
if they are born to the mothers with a history of SARS-CoV-2
infection diagnosed 14 days before or 28 days after delivery, or
if the neonate is directly exposed to close contacts with
SARS-CoV-2 infection [11]. In our study, all mothers were
positive in the third trimester, within 14 days of delivery. In
the absence of testing amniotic fluid or cord blood [12], it was
not possible to pinpoint the timing of acquisition and mode of
transmission of SARS-CoV-2 in our neonates. In our study, all
the symptomatic SARS-CoV-2 positive neonates had sepsis like
clinical presentation; it is difficult to interpret whether the
clinical course was more influenced by sepsis or SARS-CoV-2.
Till date, no published data on long term
outcome of SARS-CoV-2 recovered neonates are available with
which our findings may be compared. A multicenter cohort study
from 11 hospitals in Massachusetts described short term follow
up of 151 newborns born to SARS-CoV-2 positive mothers, till 30
days of hospital discharge although growth, neurodevelopment
were not incor-porated [13]. In this study, four babies were
re-hospitalised, due to laryngomalacia, hyperbilirubi-nemia,
ventricular arrhythmia and blood culture positive sepsis,
respectively, none directly associated with SARS-CoV-2 infection
[13]. Another follow up study from New York showed follow up
till day 25 in 23 out of 101 babies born to SARS-CoV-2 positive
mothers [14], 4 having readmissions, 3 for fever and 2 for
hyperbilirubinemia, none having evidence of SARS-CoV-2
reinfection. Several follow-up studies since the previously
known pathogenic corona viral infection outbreak - SARS
(2002-2003) are there. The outcomes in children up to 6 months
after SARS disease onset, in terms of exercise tolerance,
pulmonary function and psychologic status, have been favorable
[5,6]. All children post-SARS were found to remain clinically
asymptomatic till next 6 month; although, with mild obstructive
or restrictive defect on pulmonary function study in 10% of them
[15]. Pulmonary function test could be done in our cohort later
in life.
The limitations of this study was that only
illness severe enough to require hospital admission was
considered, which may have left out morbidities like fever,
cough and cold controlled with over the counter medicines.
Moreover, the person assessing the neurodevelopment was not
blinded to the group-assignment. Despite these shortcomings, we
may reasonably conclude that there are no differences in growth,
neurodevelopment, and hospital readmission in early infancy
between SARS-CoV-2 positive and negative neonates born to
SARS-CoV-2 positive mothers.
Ethics clearance: Institutional Ethics
Committee of Medical College Kolkata; No.
MC/KOL/IEC/NON-SPON/1046/02/2021, dated February 20, 2021.
Contributors: SR: substantial
contribution in acquisition, analysis of data, drafting the
work; DM: substantial contribution in design of the work,
interpreting the data, revising it critically for important
intellectual content; RD: substantial contribution in
acquisition of data, interpretation of results and critical
revision of the work; AH: substantial contribution in
conception, analysis of data, critical revision of the work. All
authors approved the final version to be published.
Funding: None; Competing interest:
None stated.
|
WHAT THIS STUDY ADD?
There is no difference in growth
and neurodevelopment, and rate of hospital readmission
in early infancy among SARS-CoV-2 positive and negative
neonates born to mothers with perinatal SARS-CoV-2
infection.
|
REFERENCES
1. Schwartz DA, Graham AL. Potential
maternal and infant outcomes from Coronavirus 2019-nCoV
(SARS-CoV-2) infecting pregnant women: Lessons from SARS,
MERS, and other human coronavirus infections. Viruses.
2020;12:194.
2. Dhir SK, Kumar J, Meena J, Kumar P.
Clinical features and outcome of SARS-CoV-2 infection in
neonates: A systematic review. J Trop Pediatr.
2020;28:fmaa059.
3. Raschetti R, Vivanti AJ,
Vauloup-Fellous C, et al. Synthesis and systematic review of
reported neonatal SARS-CoV-2 infections. Nat Commun.
2020;11:5164.
4. National Neonatology Forum (NNF)
COVID-19 Registry Group. Outcomes of neonates born to
mothers with coronavirus disease 2019 (COVID-19) - National
Neonatology Forum (NNF) India COVID-19 Registry. Indian
Pediatr. 2021:58:525-31.
5. Leung CW, Kwan YW, Ko PW, et al.
Severe acute respiratory syndrome among children. Pediatrics.
2004;113:e535-43.
6. Li AM, Chan CH, Chan DF. Long-term
sequelae of SARS in children. Paediatr Respir Rev.
2004;5:296-99.
7. Chawla D, Chirla D, Dalwai S, et al.
Perinatal-Neonatal Management of COVID-19 infection -
Guidelines of the Federation of Obstetric and Gynaecological
Societies of India (FOGSI), National Neonatology Forum of
India (NNF), and Indian Academy of Pediatrics (IAP). Indian
Pediatr. 2020;57:536-48.
8. World Health Organization. New WHO
growth charts. Accessed on May 26, 2021. Available from:
https://www.who.int/nutgrowthdb/software/en/
9. Alpern GD. Developmental profile 3
(DP-3). Western Psychological Services; 2007.
10. Amiel-Tison C. Neurological
evaluation of the maturity of newborn infants. Arch Dis
Child. 1968;43:89-93.
11. Wang L, Shi Y, Xiao T, et al. Chinese
expert consensus on the perinatal and neonatal management
for the prevention and control of the 2019 novel coronavirus
infection (First edition). Ann Transl Med. 2020;8:47-57.
12. Goh XL, Low YF, Ng CH, Amin Z, Ng
YPM. Incidence of SARS-CoV-2 vertical transmission: A
meta-analysis. Arch Dis Child Fetal Neonatal Ed.
2021;106:112-3.
13. Angelidou A, Sullivan K, Melvin PR et
al. Association of Maternal Perinatal SARS-CoV-2 infection
with neonatal outcomes during the COVID-19 pandemic in
Massachusetts. JAMA Netw Open. 2021;4:e217523.
14. Dumitriu D, Emeruwa UN, Hanft E et
al. Outcomes of neonates born to mothers with severe acute
respiratory syndrome coronavirus 2 infection at a large
medical center in New York City. JAMA Pediatr.
2021;175:157-67.
15. Li AM, So HK, Chu W et al. Radiological and pulmonary
function outcome of children with SARS. Pediatr Pulmonol.
2004;38:427-33.
|
|
|
 |
|

