|
|
|
Indian Pediatr 2021;58:846-849 |
 |
Long-Term Morbidity and Functional Outcome
of Japanese Encephalitis in Children: A Prospective Cohort
Study
|
|
Abhijit Dutta, 1
Shankha
Subhra Nag,1 Manjula Dutta,2
Sagar Basu3
From 1Department of Pediatric Medicine, North Bengal Medical College
and Hospital, Sushruta Nagar, Siliguri; 2Department of Microbiology,
School of Tropical Medicine, Kolkata; and 3Department of Neurology, KPC
Medical College and Hospital, Kolkata; West Bengal.
Correspondence to: Dr Shankha Subhra Nag, Embee Fortune, Flat No.
D3H, Near BSF Camp, Asian Highway 2, Kadamtala, Siliguri 734 011, West
Bengal.
Email: [email protected]
Received: December 10, 2020;
Initial review: January 27, 2021;
Accepted: May 15, 2021
Published online: May 20, 2021;
PII:S097475591600327
|
Objective: To describe the long term morbidity and functional
outcome of Japanese encephalitis in children. Methods:
Laboratory-confirmed Japanese encephalitis cases were enrolled in the
study from January, 2016 to September, 2017 and surviving cases were
prospectively followed up for 2.5 years to document various morbidities.
Outcome was functionally graded at discharge and during follow-up using
Liverpool outcome score. Results: Out of 56 children enrolled, 10
(17.9%) died during hospital stay; severe sequelae was observed in 17
(30.4%) at discharge. At the end of study, among 37 children under
follow-up, 23 (62.2%) recovered fully, 2 (5.4%) showed minor sequelae, 3
(8.1%) had moderate sequelae, and 9 (24.3%) were left with severe
sequelae. Common long term morbidities were abnormal behavior (n=10,
27%), post encephalitic epilepsy (n=8, 21.6%), poor scholastic
performance (n=8, 21.6%) and residual motor deficit (n=7,
18.9%). Improvement of morbidities was noted mostly within initial 1
year of follow-up. Conclusions: More than half of the Japanese
encephalitis survivors recovered fully, most within the first year after
discharge.
Keywords: Dystonia, Epilepsy, Movement disorder, Quadriplegia.
|
|
J apanese encephalitis
is considered a major public health problem due to its epidemic
potential, high case fatality rate up to 30%, and residual neuro-psychiatric
morbidities in 30-50% [1]. It continues to occur in endemic
areas of India, despite the introduction of the vaccine in
Universal immunization program in 2011 [2-4]. Quantification of
long term outcome and its classification in terms of extent of
disability is essential, so that the impact of the disease on
independent livelihood can be understood. There is paucity of
data regarding long term outcome of JE in children [5-7].
Therefore, this study was conducted to find out the magnitude of
morbidity and its evolution over time.
METHODS
This prospective cohort study was conducted
from January, 2016 to March, 2020 at a tertiary care teaching
hospital of eastern India, after obtaining clearance from the
institutional ethics committee. Children aged up to 12 years
admitted with acute encephalitis syndrome (AES) were subjected
to laboratory tests for detection of JE. Anti-JE IgM antibody
capture (MAC) ELISA was performed on cerebrospinal fluid and
serum samples using ELISA kit (NIV JE IgM Capture ELISA Kit,
version 1.5). Diagnosis of JE was confirmed by detection of
anti-JE IgM antibody in cerebrospinal fluid (CSF), or both in
CSF and serum samples. Patients with positive results were
consecutively enrolled till September, 2017, after taking
informed consent from parents. They were managed as per standard
guideline including empirical broad spectrum antibiotics and
acyclovir, maintenances of fluid, electrolyte, acid-base balance
and euglycemia, management of raised intracranial pressure,
control of seizures, management of nosocomial infection and
other complications, and rehabilitation therapy [8]. Background
demographic and relevant clinical data and results of various
laboratory investigations including magnetic resonance imaging
(MRI) of brain were noted. Discharged patients were followed up
for two-and-a-half years at out-patient department and detailed
clinical examination was done to document clinical status. They
were provided with symptomatic and supportive management during
these visits. EEG was performed in children with history of
seizures either during hospital stay or during follow-up. After
documentation of full recovery, patients were kept under
telephonic follow-up till the end of the study.
Table I Morbidity Profile in Children With Japanese Encephalitis at Various Stages of Follow-up
| Morbidity |
At discharge (n=46) |
6 mo (n=38) |
1 y (n=37) |
2 y 6 mo (n=37) |
| Motor deficit |
21 (45.6) |
10 (26.3) |
7 (18.9) |
7 (18.9) |
|
Abnormal behaviora |
- |
18 (47.4) |
16 (43.2) |
10 (27.0) |
| Epilepsy |
18 (39.1) |
12 (31.6) |
12 (32.4) |
8 (21.6) |
|
Poor scholastic performanceb |
- |
12 (31.6) |
8 (21.6) |
8 (21.6) |
| Incoordination |
13 (28.3) |
2 (5.3) |
2 (5.4) |
2 (5.4) |
| Feeding problems |
13 (28.3) |
3 (7.9) |
3 (8.1) |
3 (8.1) |
| Dystonia |
12 (26.1) |
6 (15.8) |
2 (5.4) |
2 (5.4) |
| Dysarthria |
7 (15.2) |
2 (5.3) |
2 (5.4) |
2 (5.4) |
| Language difficulty |
9 (19.6) |
2 (5.3) |
2 (5.4) |
2 (5.4) |
| Urinary
incontinence |
5 (10.9) |
2 (5.3) |
2 (5.4) |
2 (5.4) |
| All values in no. (%).
Evaluation started at a1 mo or b3 mo of discharge. |
The Liverpool Outcome Score (LOS) [9,10],
previously validated in Indian children [11], was used in the
present study for functional grading of disability at discharge
and during follow-up. It assesses motor, cognition, self-care
and behavior using ten questions to parents or caregivers, and
observation of response to five simple motor tasks given to the
child. Outcome grading was assigned based on minimum score
obtained in any of the domains. Based on score obtained, LOS
classifies outcome as full recovery, minor sequelae, moderate
sequelae, severe sequelae, and death.
Statistical analysis: Descriptive
statistics were used. Data were analyzed using IBM Statistical
Package for Social Sciences version 20.0 (SPSS, IBM Corp).
RESULTS
A total of 194 children with features of AES
were screened, and 56 (28.8%) children (57.1% boys) were
diagnosed with laboratory confirmed JE during the study period.
Anti-JE IgM was detected in both CSF and serum in 44 children,
and in only CSF in another 12 children. Two children were below
1 year of age, 17 between 1-5 years and the rest between 5-12
years age group; median (IQR) age of study population was 6 year
3 month (5 year 10 month, 9 year 1 month). Most common clinical
features were fever (n=56, 100%), altered sensorium (n=51,
91.1%), seizures (n=36, 64.3%), signs of meningeal
irritation (n=27, 48.2%) and headache (n=21,
37.5%). Glasgow Coma Scale of 8 or less was observed among 12
children (21.4%) at admission. Median (IQR) duration of symptoms
before admission and duration of hospitalization was 3.5 (2,5)
days and 15.7 (11, 24.2) days, respectively. MRI of brain could
be performed in 38 children, of which 26 (68.4%) were abnormal.
Common sites of involvement were thalamus (n=22, 84.6%),
basal ganglia (n=16, 61.5%), cortex (n=12, 46.2%),
brainstem (n=9, 34.6%), medial temporal lobe (n=6,
23.1%) and cerebellum (n=2, 7.7%). Hemorrhagic lesion was
found in 3 children (11.5%) in addition to involvement of other
parts of brain; 2 had cerebral hemorrhage and 1 had subdural
hemorrhage. Ten cases (17.9%) died during the hospital stay. At
the time of discharge, 17 children (30.4%) had severe sequelae,
5 (8.9%) had moderate sequelae, 6 (10.7%) developed minor
sequelae, and 18 children (32.1%) showed full recovery as per
LOS.
At the end of 2 year 6 month of follow-up, we
observed full recovery among all children with minor sequelae.
Two out of 5 children categorized as moderate sequelae at the
time of discharge showed full recovery; 1 child improved and had
only minor sequelae. Two children with severe sequelae died
within 2 weeks of discharge. Of the 15 surviving patients with
severe sequelae, one improved considerably and had only minor
sequelae, three improved and were categorized as moderate
sequelae, and nine children remained as severe sequelae. Three
fully recovered children, and two children each from moderate
and severe sequelae group were lost to follow-up. At the
completion of the study, it was observed that among 37 children
remaining under follow-up, 23 (62.2%) had recovered fully and 14
(37.8%) were left with variable degrees of sequelae (Fig. 1).
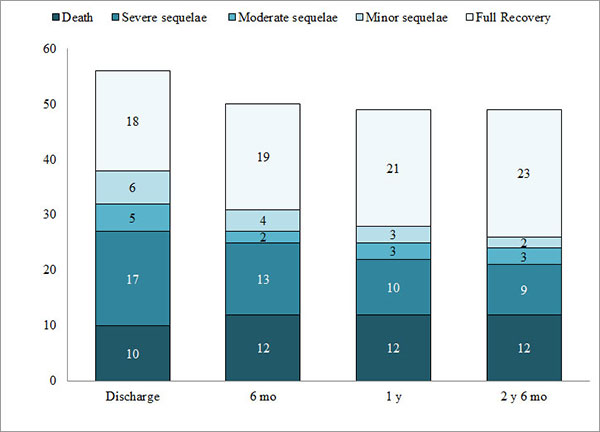 |
|
Fig. 1 Sequelae at different
stages of follow-up in Japanese encephalitis affected
children.
|
Motor deficit was noted in 21 children
(45.6%) at discharge; quadriparesis in 14, hemiparesis in 6, and
monoparesis in one child. With rehabilitation therapy,
satisfactory motor improvement was noted in majority of children
(66.7%) within the first year of follow-up. Behavioral
abnormalities evolved fully at 1 month of discharge and were
noted among 24 children; predominant features were excessive
anger (n=12), irritability (n=8), aggressiveness (n=5),
sudden bouts of unexplained cry or laughter (n=3), and
irrelevant talking (n=1). Four children were unable to
recognize family members initially, and the problem persisted in
one of them. At the end of follow-up, abnormal behavior
persisted in 10 (27%) children.
In the acute phase of the disease, 36
children presented with seizures, mostly of generalized tonic-clonic
type; 17 of them needed two or more anti-epileptic drugs (AEDs).
EEG was performed in 26 children, 18 (69.2%) were abnormal.
These children were having recurrent seizures and AED was
continued during follow-up. Among 12 children receiving AEDs at
2 years, therapy was stopped in six as they were seizure free
with normal EEG, but two children had relapse of seizures after
stoppage of drugs, and therapy was restarted. Till the end of
the study, 8 children (21.6%) were on AED.
Among cases under follow-up, 23 children were
school-going. Poor scholastic performance was observed in 8
(21.6%) children in the long term; another 7 of them became
drop-outs due to motor deficits, behavioral problems and
apprehension of seizures. Dystonia was noted in 12 children
(26.1%) at the time of discharge, which improved substantially
within first 6-9 months. Two children showed persistence of
language problem, one with motor aphasia and another with global
aphasia. Feeding problems were seen predominantly in first 6
months of follow-up; mostly due to motor deficit,
in-coordination and abnormal behavior.
DISCUSSION
In the present study, 56 children diagnosed
with Japanese encephalitis were evaluated by Liverpool Outcome
Score which showed mortality of 17.9% and 30.4% with severe
sequelae at discharge. At the end of 2.5 year follow-up, 62.2%
children recovered fully and 37.8% children were left with
variable degree of sequelae.
Previous studies have reported a wide range
of mortality (8-25%) and severe sequelae (11-25%) with Japanese
encephalitis [5,6,12-15]. Subjective nature, and therefore lack
of uniformity of classification, and varying duration of
follow-up may be responsible for wide range of sequelae noted in
different studies. The various morbidities observed in our
cohort are in agreement with previous studies [5-7,13,14]. The
extent of improvement among different morbidities varied in our
study population. Few patients with poor clinical and
radiological features showed unexpected remarkable improvement
during follow-up. Whereas some survivors with severe sequelae
showed improvement of different morbidities; nevertheless they
could not be placed at better functional grading as some other
domains did not improve. In addition, residual neuro-psychiatric
problems prevented a significant proportion of children from
returning to normal life.
Only few studies have described long term
outcome of Japanese encephalitis affected children beyond 1
year, most probably due to remote residence of patients causing
difficulty in follow-up [5-7]. Improvement of morbidities was
noted mostly within initial 9-12 months of follow up, and there
was no noteworthy additional improvement afterwards. Previous
studies also shown majority of improvements within initial 6-12
months for most of the Japanese encephalitis survivors and neuro-logical
status at initial months of discharge was predictive of long
term outcome [5,6]. Some authors noted neurological
deterioration (microcephaly and hyper-active behavior) several
years after discharge in some survivors and suggested the need
for long term follow up [6]. However, we did not observe
worsening of neuro-psychiatric status in any child till the end
of follow up.
Relatively smaller sample size is a
limitation of the present study. Similarly, we were unable to
predict which category of children might improve and to what
extent. We suggest future studies to look into these aspects.
Considering high mortality and long term morbidities, preventive
aspects of the disease need to be prioritized.
Acknowledgements: Dr Sharmistha
Bhattacherjee, Department of Community Medicine, North Bengal
Medical College and Hospital, for helping with study design and
statistical analysis.
Ethics clearance: Institutional
Ethics Committee, North Bengal Medical College; No:
PCM/2015-16/603BK, dated December 30, 2015.
Contributors: AD: conception of the
study, acquisition of data and revising the manuscript for
important intellectual content. SSN: design of the study,
acquisition of data and drafting the manuscript; MD: acquisition
of data revising the manuscript for important intellectual
content; SB: interpretation of data and revising the manuscript
for important intellectual content. All the authors approved the
version to be published and agreed to be accountable for all
aspects of the work ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
|
WHAT THIS STUDY ADDS?
•
Nearly two-thirds of
survivors of Japanese encephalitis recover without any
sequelae.
•
Common long term morbidities observed are residual
motor deficits and abnormal behavior.
|
REFERENCES
1. World Health Organization. Japanese
Encephalitis. Accessed October 10, 2020. Available from:
https://www. who.int/news-room/fact-sheets/detail/japanese-encephalitis
2. Directorate of National Vector Borne
Disease Control Programme- Delhi. State wise number of
AES/JE Cases and Deaths from 2014-2020 (till Sept). Accessed
October 10, 2020. Available from: https://nvbdcp.gov.in/WriteRead
Data/l892s/ 50863224041601897122.pdf
3. Gurav YK, Bondre VP, Tandale BV, et
al. A large outbreak of Japanese encephalitis predominantly
among adults in northern region of West Bengal, India. J Med
Virol. 2016; 88:2004-11.
4. Vashishtha VM, Ramachandran VG.
Vaccination policy for Japanese encephalitis in India: Tread
with caution! Indian Pediatr. 2015;52:837-9.
5. Baruah HC, Biswas D, Patgiri D, et al.
Clinical outcome and neurological sequelae in serologically
confirmed cases of Japanese encephalitis patients in Assam,
India. Indian Pediatr. 2002;39:1143-8.
6. Ooi MH, Lewthwaite P, Lai BF, et al.
The epidemiology, clinical features, and long-term prognosis
of Japanese encephalitis in central Sarawak, Malaysia,
1997-2005. Clin Infect Dis. 2008;47:458-68.
7. Ding D, Hong Z, Zhao SJ, et al.
Long-term disability from acute childhood Japanese
encephalitis in Shanghai, China. Am J Trop Med Hyg.
2007;77:528-33.
8. Sharma S, Mishra D, Aneja S, Expert
Group on Encephalitis, Indian Academy of Pediatrics.
Consensus Guidelines on Evaluation and Management of
Suspected Acute Viral Encephalitis in Children in India.
Indian Pediatr. 2012;49:897-910.
9. University of Liverpool Brain
Infections Group. Liverpool Outcome Score for Assessing
Children at Discharge. Accessed October 10, 2020. Available
from: https:// vaccineresources.org/details.php?i=676#:~:text=A%20
tool%20for%20doctors%20and,independent%20life%20
after%20Japanese%20encephalitis
10. University of Liverpool Brain
Infections Group. Liverpool Outcome Score for Assessing
Children at Follow–up. Accessed October 10, 2020. Available
from: https://vac cineresources.org/details.php?i=677.
11. Lewthwaite P, Begum A, Ooi MH, et al.
Disability after encephalitis: Development and validation of
a new outcome score. Bull World Health Organ.
2010;88:584-92.
12. Basumatary LJ, Raja D, Bhuyan D, et
al. Clinical and radiological spectrum of Japanese
encephalitis. J Neurol Sci. 2013;325:15-21.
13. Kakoti G, Dutta P, Ram Das B, et al.
Clinical profile and outcome of Japanese encephalitis in
children admitted with acute encephalitis syndrome. Biomed
Res Int. 2013;2013: 152656.
14. Maha MS, Moniaga VA, Hills SL, et al.
Outcome and extent of disability following Japanese
encephalitis in Indonesian children. Int J Infect Dis.
2009;13:e389-93.
15. Verma A, Tripathi P, Rai N, et al. Long-term outcomes and
socioeconomic impact of japanese encephalitis and acute
encephalitis syndrome in Uttar Pradesh, India. Int J Infect.
2017; 4:e15607.
|
|
|
 |
|

