|
|
|
Indian Pediatr 2021;58: 826-832 |
 |
Add-on Home-Centered Activity-Based
Therapy vs Conventional Physiotherapy in Improving Walking
Ability at 6-Months in Children With Diplegic Cerebral Palsy:
A Randomized Controlled Trial
|
|
Jyotindra Narayan Goswami, Naveen Sankhyan, Pratibha
Singhi
From Pediatric Neurology and Neurodevelopment Unit,
Advanced Pediatrics Centre, Post Graduate Institute of
Medical Education and Research, Chandigarh;
Correspondence to: Prof Pratibha Singhi, Director,
Department of Pediatrics Neurology and Neurodevelopment,
Medanta, The Medicity, Gurgaon, Haryana.
Email:
[email protected]
Received: June 05, 2019;
Initial review: October 09, 2019;
Accepted: May 26, 2021.
Published online: May 28, 2021;
PII: S097475591600332
Clinical Trial Registration No. : NCT02412007
|
|
Background:
Institutional physiotherapy as a standard of care for management
of cerebral palsy (CP) has certain shortcomings, especially in
resource-constrained settings. This is a proof-of-concept trial
to evaluate the efficacy of individualized home-centered
activity-based therapy in children with spastic diplegic CP.
Design: Randomized
controlled trial (open-label).
Settings:
Tertiary-care hospital with pediatric neurology services (July,
2014 to July, 2016).
Participants:
Consecutive sample of 59 children (5-12 y) with spastic diplegic
CP (Gross Motor Function Classification System scores II-III)
without fixed lower-limb contractures, illnesses impeding
physiotherapy or history of recent botulinum toxin
injection/surgery were recruited.
Procedure: Children
were randomized to Intervention or Control arms. Their
6-minute-walk Test (6MWT) scoring and clinical examination were
performed at baseline, 3 and 6 months. Children in Intervention
arm (n=30) were prescribed parent-supervised
home-centered activity-based therapy (walking, standing,
squatting, climbing upstairs/downstairs, kicking a ball,
dancing, riding a tricycle/bicycle) in addition to their
institutional physiotherapy. Children in Control arm (n=29)
were prescribed ongoing institutional physiotherapy alone.
Logbooks, home videos and telephonic follow-ups were used to
ensure compliance.
Main outcome measures:
Comparison of the mean change in 6MWT scores at 6 months
(from baseline) between the two groups.
Results: Median (IQR)
change in 6MWT scores at 6 months (from baseline) in the
Intervention and Control arms were 3.5 (-5.3, 9) m and 3 (-7.8,
6.3) m
Conclusions: Adjunct
home-centered activity-based therapy was safe and feasible, but
did not result in appreciable gains over 6 months.
Keywords: Neurorehabilitation,
Play therapy, Brain injury, Perinatal brain injury.
|
P
hysical therapy is
the cornerstone in the
management of cerebral palsy (CP). Various
targets of physical therapy include spasticity-reduction, functional mobility optimization and prevention
of secondary musculoskeletal complications [1]. Numerous
schools of physical therapy are based on different
principles, and have their own pros and cons [2]. Existence
of a multitude of physical therapy techniques indicates that
a singularly effective regime for children with CP is yet to
be formulated. Apart from physical therapy, holistic
rehabilitative practices aim to improve affected
individuals’ functional capabilities so that they may
gainfully participate in day-to-day activities [3].
Activity-based therapy is generating
interest as an alternative mode of CP rehabilitation [4].
The philosophy behind this modality is functional
improvement through repetitive performance of activity-based
training, lifestyle modifications, and mobility-enhancing
devices rather than traditional passive physiotherapy
protocols. Activity-based rehabilitative regimes that have
been scientifically evaluated and found effective include
Constraint-Induced Movement Therapy (CIMT) (for hemiparetic
CP rehabilitation), treadmill therapy (for gait disorders)
and Robotic arm technique. For conventional physical
therapy, the deficiency of physiotherapists, equipment and
rehabilitation centers are limiting factors in
resource-limited settings [6]. A simple home-based regime
empowers parents and truncates expenses of institutional
care [7]. The role of home-centered, activity-based therapy
in children with diplegic CP has not been evaluated till
date. Our study was designed as a proof-of-concept trial to
look into the efficacy of home-centered activity-based
therapy in children with spastic diplegic CP in
resource-limited settings.
METHODS
This randomized controlled trial (RCT)
was conducted in a tertiary-level pediatric teaching
hospital and its associated rehabilitation centre from July,
2014 to July, 2016 after obtaining approval from the
institutional ethics committee.
Children between 5 and 12 years of age,
clinically diagnosed with spastic diplegic CP with Gross
Motor Function Classification System (GMFCS) Score II/ III
were eligible. Spastic diplegic CP was defined as ‘CP with
predominant bilateral lower limb involvement with
hyperreflexia, spasticity and relative non-involvement of
upper limbs’. Enrolled children required a minimum visual
acuity of 6/60 and the ability to follow single-step
commands. They were enrolled if their parents/primary
caregivers were willing and capable of following
instructions and maintaining an activity log. Informed
consent from the parent(s)/primary caregiver(s) and assent
(as and when applicable, from a child) were obtained before
enrolment. Children with fixed lower-limb contractures or
deformities affecting stance and gait, chronic
systemic/acute illnesses interfering with physiotherapy and
children who received botulinum toxin injection or underwent
corrective orthopedic surgery up to one year prior to the
day of screening were excluded.
A detailed general and systemic
examination of every child was performed and findings
recorded on proforma. Each child was scored for
6-minute-walk-test (6MWT) (in meters),
10-metre-fast-walk-test (10MFWT)(in seconds), modified
Ashworth scale (MAS), modified Tardieu scale (MTS), Gross
Motor Function Classification System (GMFCS), Gross Motor
Function Measure-88 (GMFM-88) (D & E) and Cerebral Palsy
Quality of Life (CP-QoL) (Primary-caregiver).
Randomization was done by another person
who was otherwise not involved in any other aspect of the
trial. Block randomization of varying sizes was prepared
with an open access randomization software (www.
randomizer.org). Allocation concealment was achieved
using sealed opaque envelopes. Envelopes were kept in
custody of a person not involved in the study.
Scoring for 6MWT: Test arena
comprised of a marked, flat, non-slippery, rectangular
cemented area with a perimeter of 34 meters. Children were
made to walk along the outer boundary of the area after an
initial demonstration. If at any point, the child had
difficulty walking (due to any reason) or did not want to
walk, he or she could stop. In that case, the test would be
postponed to a later date. Practice trial immediately
preceding test was avoided to reduce fatigue [8]. The
parents/primary caregivers were instructed to encourage the
child throughout the process. In case a child needed
one-hand support, the parent/primary caregiver walked
alongside the child holding his/her hand. Distance covered
in 6 minutes was recorded in meters (up to nearest
centimeter). The test was videographed for future reference.
Scoring for 10MFWT: Test area
comprised of a cemented, flat, non-slippery, rectangular
floor. Start and finish lines were marked with parallel
white lines 10m apart. An explanation and demonstration
preceded the test. To start, the child was made to stand
with toes touching the start-line. On being told to start,
the child had to start walking. The stop command was given
after the child had walked 5m past the finish line so that
he/she may not decelerate until after reaching the 10 m mark
[9]. Time to walk 10m was recorded with a stopwatch with the
least count of 0.01 seconds. The test was video graphed for
future reference.
Eight activities were recommended in the
‘Intervention Arm,’ viz., walking on plain surface, standing
from squatting position and squatting from standing position
on level floor, climbing up and down a flight of stairs,
kicking a football while standing, dancing on level floor,
riding a tricycle/bicycle (with additional support wheels)
depending on child’s age and functional capability. Every
child was expected to perform at least seven out of eight
activities (exception being cycling) to the best of his/her
ability. Parents/primary caregivers were given a brief
overview of the interventions in the language they
understood. They were taught how to keep the activities
interesting by incorporating them within play activities.
Activities were tailored according to the age and functional
capacity of the child and modified periodically to avoid
monotony. Strict compliance was emphasized upon. At the
outset, videographic demonstrations of the activities were
displayed to the parents. Videos were prepared in the same
centre previously and approved by all the researchers
involved in the study. The parents/primary caregivers were
asked to make their children perform the activities under
the researcher’s supervision. Doubts were clarified and
modifications suggested. A logbook was issued to each
parent/primary caregiver with simple instructions written in
English and Hindi and sketches depicting the activities. The
logbook had an earmarked space for every day ,in which the
parent/primary caregiver was instructed to record whether
the pre-assigned activity was performed as advised or not.
There was a ‘remarks’ column for noting any deviations.The
logbook was designed to serve as the main mechanism to
ensure compliance. At the completion of the study,
percentage compliance was calculated from the entries in the
logbooks. In addition, home videos taken by parents acted as
a reinforcement of compliance. The activities were designed
to be repeated three times per session, with three sessions
per day for five days per week. In case of any acute
illness, pain, injury, compelling domestic issues or in the
case of a child strongly resenting therapy, a break could be
allowed but the parent/primary caregiver was supposed to
record it in the logbook. These activities were in addition
to activities that were already being performed as a part of
an ongoing physiotherapy program. Parents/primary caregivers
of children in the Control arm were advised to continue
ongoing physiotherapy i.e. certain sets of active and
passive exercises that were administered by certified
physiotherapists after individualized assessment as per
guidelines. Three physiotherapists offered conventional
physiotherapy in the study centre. These manoeuvres were
tailored to the needs of a child with periodic reassessment
and modification as per the disability, tolerability of
intervention and clinical response (spasticity, stability of
gait, etc). Techniques of conventional physiotherapy are
described in Web Box I.
Besides filling the logbook, the
parents/primary caregivers also made intervention home
videos periodically, which were perused by one investigator
at follow-up. Telephonic follow-up was done at 2 weeks of
enrolment. The telephone number of the primary investigator
was shared with parents/primary caregivers for addressing
queries. In addition, parents who expressed difficulty in
understanding the procedures were contacted telephonically
to reinforce the prescribed procedures. Follow-up visits
were planned at 3 months (+ 1 week), 6 months and (+ 2
weeks) after enrolment. Doses of drugs (such as baclofen or
antiepileptics) were not modified for study purposes. If any
child developed seizures during the course of study, he/she
was managed as per standard protocol.
The primary study outcome was change in 6
MWT score at 6 months. The secondary outcomes included
change in 10MFWT score (in seconds), MAS, MTS, GMFM-88 (D &
E), CP-QoL (Primary caregiver version) scores at 3 and 6
months, and change in 6MWT score at 3 months. A single
investigator assessed all the outcomes.
For sample size calculation, it was
assumed that the intervention would result in 60 m change in
distance covered in 6MWT. This value of 60 m was an
extrapolation from the study by Fitzgerald, et al. [10]
in children with CP (GMFCS II) wherein, the standard
deviation was 77. Keeping
a as 0.05
and power of study as 80%, a sample size of 27 in each group
was required . Assuming a 10% loss to follow-up, the
targeted sample size was 59 (minimum 30 children per group).
Statistical analysis: Data were
recorded on a Microsoft Excel spreadsheet. Statistical
analysis was performed using the statistical software SPSS
(IBM Corp), Version 20. Student t-test was used to compare
the difference in the means between the two groups for
parametric variables, while non-parametric variables were
expressed as median (IQR) and compared using Mann-Whitney U
test
RESULTS
Two hundred and sixty-two children with
CP were screened, among whom 169 met inclusion criteria and
59 were enrolled (Fig. 1). Thirty children were
randomized to the Intervention arm and 29 to the Control
arm. One child allocated to the control arm withdrew from
the study.
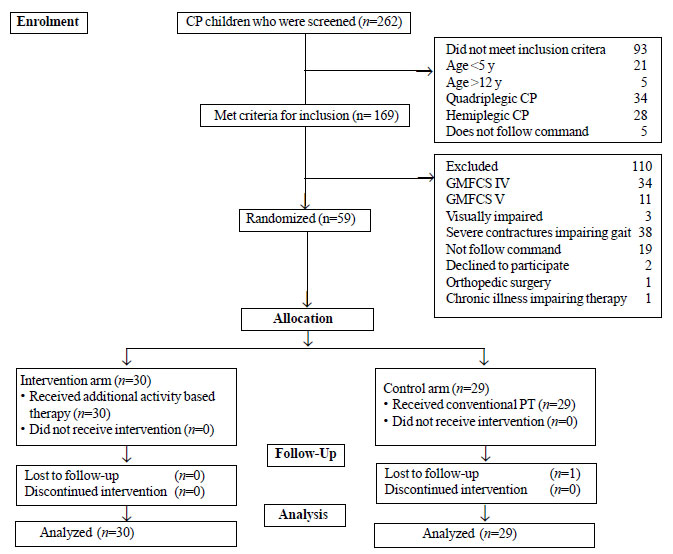 |
|
Fig. 1 CONSORT flow
diagram for the study.
|
The mean (SD) ages of children in the
study was 115 (23.1) months. Anthropometric and clinical
character-istics of the two groups were comparable at
baseline (Table I). Five children (intervention arm:
3, control arm: 2) had fresh onset seizures during the study
period in all of which oral sodium valproate was initiated.
Good seizure control was achieved in four of them with
monotherapy of sodium valproate (dose range 15-40mg/kg/day)
while one child (intervention arm) required additional
therapy with oral clobazam. Except for a brief interruption
of their respective intervention schedules, there was no
long-term interruption of the rehabilitation program in any
of these children. The majority of children in both groups
achieved a compliance rate of 80-100%, There were no
differences in the compliance rates between the children in
the two groups, as derived from logbooks filled by the
primary caregivers, with 93.3% and 93.1% children in
intervention and control group, respectively having rates
>60%.
Table I Baseline Characteristics of Children with Diplegic Cerebral Palsy in the Intervention and Control Arms
| Characteristic |
Intervention arm
|
Control arm
|
|
(n=30) |
(n=29) |
| Age, moa |
73(65-89) |
71(63-79) |
| Males |
19 (32) |
24 (41) |
| Functional status |
|
|
| GMFCS II |
16 (53) |
11 (40) |
| GMFCS III |
14 (47) |
18 (60) |
| Assistive devices |
|
|
|
Ankle-foot-orthosis
|
10 (33) |
14 (48) |
|
Knee-ankle-foot-orthosis
|
2 (7) |
2 (7) |
| Epilepsy |
8 (27) |
8 (28) |
| Refractive error |
8 (27) |
6 (21) |
|
Receiving baclofen
|
17 (57) |
20 (69) |
|
All values in no. (%) or amedian (IQR). GMFCS: Gross
motor functional classification scale. |
Table II Change in 6-Minute Walk Test (6MWT) Scores in Children With Spastic Diplegia in the Two Groups
|
Intervention arm |
Control arm |
|
(n=30) |
(n=29) |
| Baseline |
227.5 (168.8,340) |
243.0 (142.5,350) |
| 3 mo |
225.5 (165.5,343.3) |
230.0 (134.5,336) |
| 6 mo |
229.0 (165.3,340.8) |
246.0 (141,336) |
| 0-6 moa |
3.5 (-5.3, 9.0)] |
3.0 (-7.8,6.3) |
|
All values in median (IQR). aDifference between
scores at baseline and at 6 months. P>0.05 for all
comparisons. |
The difference in mean 6 MWT between
baseline and 6 months was 3.5m and 3m in the Intervention
and Control Arm, respectively (Table II). There was
no significant change in any of the secondary outcome
variables (Web Table I).
DISCUSSION
The study is a proof of concept trial to
evaluate the efficacy of a home-centered activity-based
rehabilitation program for children with diplegic CP.
Childrn with diplegia constitute a major subset of CP [11].
It is probable that children younger than 5 years would
probably have responded better owing to their
neuro-plasticity. However, they were excluded from the study
due to issues in eliciting cooperation, validity of 6MWT in
toddlers, and because activity regime would have required
significant modification in younger children.
Common interventions for physiotherapy in
CP children include strength and functional training; weight
supported treadmill training (WBSTT), and
neuro-developmental treatment (NDT) [2]. None of these
techniques are universally applicable. Children in this
study followed a standardized institutional physiotherapy
regime comprising of passive joint mobility and assisted
gait training as outlined in methodology. Activity-based
therapy refers to a regime of age-appropriate motor
activities such as walking, for example, which involves
multiple repetitions of coordinated, reciprocal limb
activities [12]. CIMT is a popular and effective example of
upper limb activity-based therapy [13-15]. Activity-based
therapy for lower limbs may include day-to-day activities
like climbing stairs, walking, and sit-to-stand. Intense
activity-based training, lifestyle modifications, and
mobility-enhancing devices are hypothesized to increase
motor activity leading to better physical and mental health
cognitive performance in people with motor impairments [12].
The neurophysiological basis of improvement rests on the
principles of neuroplasticity [4,16,17]. There is a
scientific basis to the assumption that regular activities
performed by a child with CP would lead to alteration in the
representation of the motor cortex with corresponding motor
improvement. Intensive upper limb rehabilitation has been
seen to be associated with enhanced motor area activation
and size in children with CP [18]. Brains of individuals
with CP have been noted to display adaptation in the motor
areas subsequent to rehabilitation and activity [19]. Hence,
the hypothesis of the index study appears biologically
plausible. In order to explore neuroplastic modeling,
researchers have attempted integrated neurorehabilitation
using combined modalities of physical therapy, magnetic
stimulation and nutraceuticals [20].
Simple, interesting, age-appropriate,
safe, econo-mically feasible and objectively assessable
activities within the ambit of day-to-day functioning were
incorporated in the study. Walking was a key component.
Maher, et al. [21] studied a walk-based model of
rehabilitation in children with CP between 8-17 years of age
in the Step Up study. Azizi S, et al. [22] demonstrated that
anti-gravity treadmill therapy is effective in improving
gait in CP. Squatting and standing are additional tasks
included in the activity schedule meant to increase the
strength of lower limb musculature and to promote functional
mobility. Contemporary systematic review has quoted Level II
(b) evidence in support of sit-to-stand training for
improvement of balance [23]. Climbing up and down stairs is
intended to improve functional mobility. Kicking a football
has not been reported as a specific therapeutic modality for
rehabilitation in CP. It is anticipated to increase lower
limb strength, balance, and coordination while sustaining
the child’s interest. Cycling was chosen to improve lower
limb strength and joint mobility, reduce spasticity and to
make the program joyful for the children. However, this was
reserved as an optional activity depending on the child’s
ability, interest and availability of cycle. The cycles used
were either tricycles or bicycles with two accessory
balancing wheels. The utility of different types of cycling
in neurorehabilitation has been previously reported [24,25].
Dancing has been included in order to promote joint mobility
and balance while maintaining the child’s interest in the
program, as previously reported [26,27]. However, in the
present study, the dancing activity was an unstructured one.
In the present study, activity-based interventions were
chosen so that the children could perform them either
independently or with minimal assistance. So children with
functional levels of GMFCS IV-V were excluded from our
study.
The role of task-directed rehabilitation
is evident from contemporary upper limb rehabilitation
programs [29]. There is a growing body of evidence of
molecular plasticity and functional recovery secondary to
CIMT [30]. The test-test reliability and validity of 6MWT in
children and adolescents with CP has been previously
demonstrated [31,32]. A timeline of 6 months for measuring
the primary outcome was adopted in our study arbitrarily as
it was anticipated that there would be some change in the
functional status of the children by that period without
suffering significant attrition. Home-based rehabilitation
is exalted in view of benefits such as better compliance,
involvement and empowerment of parents, economical and
feasible [33]. The greatest strength of the study is that it
highlights the feasibility of a home-based rehabilitation
program in resource-constrained settings. This family-based
model simplistic model is appealing because it is
economically viable and suits the needs of
resource-constrained settings. However, the comparable
difference in 6MWT at 6 months (from baseline) in the two
groups indicates the fact that adjunctive home-centered
activity-based therapy does not improve the outcome
associated with regular institutional physiotherapy. The
initial 3-month decline and the latter 3-month improvement
noted in the study were higher in the Control arm. This
phenomenon may be probably due to fewer fluctuations in 6MWT
in children receiving both home-based and institutional
therapy due to a stable trajectory. It is unlikely that
compliance issues could explain the differential trends
because compliance was stable across the study in both
groups. Satisfactory adherence was maintained throughout the
study indicating that, if applied in the community, this
model is likely to be well accepted. There was no
intervention-related adverse effects eliciting the safe
nature of the regime.
The results do not show a significant
difference between the two groups probably because of
certain limitations in the study design such as brief
follow-up period, low intensity of interventions,
Parent/primary caregiver report-based compliance assessment
and varied etiologies of CP. There was no mechanism in the
study, which could ensure absolute uniformity in the
administration of interventions and live monitoring of the
same. A feasible approach to compliance monitoring was
adopted at the cost of increasing bias. Despite concealed
group allocation, follow-up and evaluation were open-label
with the potential risk of bias. The novelty of the study
lies in its practical model whereby a simplistic
home-centered program, with day-to-day activities, have been
analyzed in children with diplegic CP. Hence no analytical
comparison to other similar studies could be drawn.
Our study revealed that home-centred
activity-based therapy is a feasible and practical modality
of CP rehabilitation; however, significant benefits were not
appreciable over a 6-month period, therefore, reinforcing
need for intense institutional-based therapy. We suggest a
larger study size with more intensive intervention strategy,
prolonged follow-up interval and more stringent compliance
monitoring be conducted in order to effectively evaluate the
efficacy of home-centered activity-based programme in
children with CP.
Acknowledgments: Mrs. Naresh Kumari,
Physiotherapist for her assistance during the trial.
Ethics clearance: Institute Ethics
Committee (Intramural), PGIMER, Chandigarh; Histopath/14/3667,
dated September 24, 2014.
Contributors: JNG: patient
management, data collection, literature review, and
preparation of the draft manuscript; NS: Protocol
development, supervision of the study, interpretation of
results, editing and final approval of the manuscript. PS:
Concept and design of the study, supervising conduct of the
study, interpretation of results, clinician-in-charge of
patient manage-ment and final approval of the manuscript.
Funding: None; Competing interest:
None stated.
Note: Additional material related to
this study is available with the online version at
www.indianpediatrics.net. Presented at the 14th Asian
and Oceanic Congress of Child Neurology, May 11-15, 2017,
Fukuoka, Japan.
|
WHAT IS ALREADY KNOWN?
•
Physiotherapy plays a major
role in the management of children with cerebral
palsy.
WHAT THIS STUDY ADDS?
•
Adjunctive
home-centred activity-based therapy does not improve
the functional outcomes of children with CP as
measured by 6-Minute-Walk-Test scores at 6 months
when compared with those receiving institutional
physiotherapy alone.
|
REFERENCES
1. Castelli E, Fazzi E.
Recommendations for the rehabilitation of children with
cerebral palsy. Eur J Phys Rehabil Med. 2016;52:691-703.
2. Martin L, Baker R, Harvey A. A
systematic review of common physiotherapy interventions
in school-aged children with cerebral palsy. Phys Occup
Ther Pediatr. 2010;30:294-312.
3. Vargus-Adams JN, Majnemer A.
International Classification of Functioning, Disability
and Health (ICF) as a framework for change
revolutionizing rehabilitation. J Child Neurol.
2014;29:1030-5.
4. Cao J, Khan B, Hervey N, et al.
Evaluation of cortical plasticity in children with
cerebral palsy undergoing constraint-induced movement
therapy based on functional near-infrared spectroscopy.
J Biomed Opt. 2015;20: 046009.
5. Diwan S, Diwan J, Bansal AB, Patel
PR. Changes in capacity and performance in mobility
across different environmental settings in children with
cerebral palsy: An exploratory study. J Clin Diagn Res.
2015;9:1-3.
6. Kumar SG, Roy G, Kar SS.
Disability and rehabilitation services in India: Issues
and challenges. J Fam Med Prim Care. 2012;1:69-73.
7. MOB Olaogun, GGG Nyante, AI
Ajediran. Overcoming the barriers for participation by
the disabled: An appraisal and global view of
community-based rehabilitation in community development.
African Journal of Physio-therapy and Rehabilitation
Sciences. 2009;1:24-9.
8. ATS Committee on Proficiency
Standards for Clinical Pulmonary Function Laboratories.
ATS Statement: Guidelines for the Six-Minute Walk Test.
Am J Respir Crit Care Med. 2002;166:111-7.
9. Watson MJ. Refining the ten-metre
walking test for use with neurologically impaired
people. Physiotherapy. 2002;88:386-97.
10. Fitzgerald D, Hickey C, Delahunt
E, Walsh M, O’Brien T. Six-minute walk test in children
with spastic cerebral palsy and children developing
typically. Pediatr Phys Ther. 2016;28:192-9.
11. Singhi P, Saini AG. Changes in
the clinical spectrum of cerebral palsy over two decades
in North India–an analysis of 1212 cases. J Trop Pediatr.
2013;59:434-40.
12. Damiano DL. Activity, activity,
activity: rethinking our physical therapy approach to
cerebral palsy. Phys Ther. 2006;86:1534-40.
13. Choudhary A, Gulati S, Kabra M,
et al. Efficacy of modified constraint induced movement
therapy in improving upper limb function in children
with hemiplegic cerebral palsy: a randomized controlled
trial. Brain Dev. 2013;35:870-6.
14. Dong VA, Fong KNK, Chen Y-F,
Tseng SSW, Wong LMS. "Remind-to-move" treatment versus
constraint-induced movement therapy for children with
hemiplegic cerebral palsy: A randomized controlled
trial. Dev Med Child Neurol. 2016;59:160-7.
15. Whitehead L. Constraint-induced
movement therapy for hemiparesis following stroke. Am J
Nurs. 2016;116:63.
16. Sutcliffe TL, Logan WJ, Fehlings
DL. Pediatric constraint-induced movement therapy is
associated with increased contralateral cortical
activity on functional magnetic resonance imaging. J
Child Neurol. 2009;24:1230-5.
17. Inguaggiato E, Sgandurra G,
Perazza S, Guzzetta A, Cioni G. Brain reorganization
following intervention in children with congenital
hemiplegia: A systematic review. Neural Plasticity.
2013;356275.
18. Bleyenheuft Y, Dricot L, Gilis N,
et al. Capturing neuroplastic changes after bimanual
intensive rehabili-tation in children with unilateral
spastic cerebral palsy: A combined DTI, TMS and fMRI
pilot study. Res Dev Disabil. 2015;43-44:136-49.
19. de Almeida Carvalho Duarte N,
Collange Grecco LA, Zanon N, et al. Motor cortex
plasticity in children with spastic cerebral palsy: A
systematic review. J Mot Behav. 2017;49:355-64.
20. Novak MR. Promoting
neuroplasticity in a developing brain: Integrated
neurorehabilitation (INRA) for children with cerebral
palsy - A protocol description and case report. J Neurol
Stroke. 2016;4:00137.
21. Maher C, Crettenden A, Evans K,
Thiessen M, Toohey M, Dollman J. A pedometer based
physical activity self-management program for children
and adolescents with physical disability - design and
methods of the StepUp study. BMC Pediatr. 2014;14:31.
22. Azizi S, Marzbani H, Raminfard S,
Birgani PM, Rasooli AH, Mirbagheri MM. The impact of an
anti-gravity treadmill (AlterG) training on walking
capacity and corticospinal tract structure in children
with cerebral palsy. Conf Proc IEEE Eng Med Biol Soc.
2017;2017:1150-3.
23. Dee M, Lennon O, O’Sullivan C. A
systematic review of physical rehabilitation
interventions for stroke in low and lower-middle income
countries. Disabil Rehabil. 2018; 3:1-29.
24. Demuth SK, Knutson LM, Fowler EG.
The PEDALS stationary cycling intervention and
health-related quality of life in children with cerebral
palsy: A randomized controlled trial. Dev Med Child
Neurol. 2012;54:654-61.
25. Chen Y, Garcia-Vergara S, Howard
AM. Effect of a home-based virtual reality intervention
for children with cerebral palsy using super pop VR
evaluation metrics: A Feasibility Study. Rehabil Res
Pract. 2015;2015:812348.
26. López-Ortiz C, Gladden K, Deon L,
Schmidt J, Girolami G, Gaebler-Spira D. Dance program
for physical rehabilitation and participation in
children with cerebral palsy. Arts Health. 2012;4:39-54.
27. Morán Pascual P, Mortes Roselló
E, Domingo Jacinto A, et al. On the use of dance as a
rehabilitation approach for children with cerebral
palsy: A single case study. Stud Health Technol Inform.
2015; 217:923-8.
28. Jung SH, Song SH, Kim SD, Lee K,
Lee G. Does virtual reality training using the Xbox
Kinect have a positive effect on physical functioning in
children with spastic cerebral palsy? A case series. J
Pediatr Rehabil Med. 2018;11:95-101.
29. Plasschaert VFP, Vriezekolk JE,
Aarts PBM, Geurts ACH, Van den Ende CHM.Interventions to
improve upper limb function for children with bilateral
cerebral palsy: A systematic review. Dev Med Child
Neurol. 2019;61:899-907.
30. Liu XH, Bi HY, Cao J, Ren S, Yue
SW. Early constraint-induced movement therapy affects
behavior and neuronal plasticity in ischemia-injured rat
brains. Neural Regen Res. 2019;14:775-82.
31. Thompson P, Beath T, Bell J, et
al. Test-retest reliability of the 10-metre fast walk
test and 6-minute walk test in ambulatory school aged
children with cerebral palsy. Dev Med Child Neurol.
2008;50:370-6.
32. Maher CA, Williams MT, Olds TS.
The six-minute walk test for children with cerebral
palsy. Int J Rehabil Res. 2008;31:185-8.
33. Beckers LWME, Schnackers MLAP, Janssen-Potten YJ,
Kleijnen J, Steenbergen B. Feasibility and effect of
home-based therapy programmes for children with cerebral
palsy: A protocol for a systematic review. BMJ Open. 2017;
7:e013687.
|
|
|
 |
|

