|
|
|
Indian Pediatr 2021;58: 815-819 |
 |
Modified Atkins Diet vs
Low Glycemic Index Treatment for Drug-Resistant Epilepsy in
Children: An Open Label, Randomized Controlled Trial
|
|
Surbhi Gupta, 1 Surekha
Dabla,2 Jaya Shankar Kaushik1
From Departments of 1Pediatrics and 2Neurology, Pandit Bhagwat Dayal
Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana,
India.
Correspondence to: Dr Surekha Dabla, Senior Professor, Department of
Neurology, Pt BD Sharma Post Graduate Institute of Medical Sciences,
Rohtak, Haryana 124 001, India.
Email:
[email protected]
Received: June 06, 2020;
Initial review: July 07, 2020;
Accepted: December 10, 2020.
Published online: February 25,
2021;
PII: S097475591600297
Clinical Trial Registration: CTRI/2017/12/010898
|
|
Objective: To compare the
efficacy of the modified Atkins diet (mAD) and low glycemic index
treatment (LGIT) among children with drug-resistant epilepsy.
Design: Randomized, open
labelled, controlled clinical trial.
Setting: Tertiary care
referral center.
Participants: Children aged 6
months to 14 years with drug-resistant epilepsy.
Intervention: mAD (n=30)
or LGIT (n=30) as an add-on to the ongoing antiseizure drugs.
Main outcome measures: Proportion
of children who achieved seizure freedom as defined by complete
cessation of seizure at 12 weeks as primary outcome measure. Secondary
outcome measures were proportion of children who achieved >50% and >90%
seizure reduction at 12 weeks, and adverse effects of the two therapies.
Results: Of the 60 recruited
children, 3 in the mAD group, and 3 in LGIT group were lost to
follow-up. The proportion of children with seizure freedom [16.6% vs
6.6%; relative risk reduction (RRR) (95% CI), 1.5 (-10.9, 0.5); P=0.42]
and >90% seizure reduction [30% vs 13.3%; RRR, -1.2 (-5.5, 0.2); P=0.21]
was comparable between the mAD and LGIT group at 12 weeks. The
proportion of children with >50% seizure reduction was significantly
higher at 12 weeks among those who received LGIT as compared to the
mADgroup [73.3% vs 43.3%; RRR (95% CI) 0.4 (0.1-0.6); P=0.03]
although the effect size was small. The diet was well tolerated with
lethargy being the most common adverse effect in children in mAD (53.3%)
and LGIT (66.7%) groups.
Conclusion: The present study
with limited sample size shows that seizure freedom at 12 weeks was
comparable between mAD and LGIT for the treatment of drug-resistant
epilepsy.
Keywords: Dietary therapy, Efficacy, Ketogenic
diet.
|
|
K
etogenic dietary therapies are useful
non-pharmacological therapeutic options in the management of drug
resistant epilepsy [1]. Types of dietary therapy for epilepsy include
the classical ketogenic diet, modified Atkins diet, low glycemic index
treatment, and medium-chain triglyceride diet [2]. The classical
ketogenic diet (KD) is high-fat (80%), low protein (15%), and low
carbohydrate (5%) diet effective in drug-resistant epilepsy [3-5].
However, KD is a tedious procedure with a need for dietician as it is a
stringent diet, requires lot of calculations and weighing of the food
items, which makes it challenging to administer in resource-constrained
settings. The modified Atkins diet (mAD) is a more liberal, less
restrictive, and more palatable type of diet, which yields high
compliance and similar effectiveness as compared to classical KD [6-9].
However, compliance, and weighing of food items is a drawback of this
diet as well.
The low glycemic index treatment (LGIT) was developed
as a liberalized alternative to the KD and mAD for seizure management
[10-12]. LGIT diet includes food with a glycemic index less than 50. The
LGIT is gaining popularity for treatment for epilepsy due to its
effectiveness, mild side effect profile and more palatability. Hence,
the present study was designed with a hypothesis that the two groups
(LGIT and mAD) would not have a significant difference in seizure
control outcome.
METHODS
This open-label, randomized controlled trial was
conducted in the Departments of Pediatrics and Neurology of a public
sector tertiary-care referral center. The data were collected from
February, 2018 to March, 2019. Ethical approval from the institutional
ethics committee was obtained, and a written informed consent was taken
from the parents. Children aged six months to 14 years with
drug-resistant epilepsy (failure of adequate trials of two tolerated,
appropriately chosen anti-seizure drug schedules, whether as
monotherapies or in combination to achieve sustained seizure freedom
[13]) were enrolled. Children with known or suspected inborn error of
metabolism, systemic illness, and severe acute malnutrition were
excluded.
Eligible children were randomized to receive either
mAD or LGIT along with their ongoing conventional anti-seizure drug.
Each child was subjected to clinical history and examination. Seizure
type, frequency, age at onset, perinatal details, family history,
developmental status and treatment history was recorded. If the child
was on any syrup formulation, it was converted to tablets to avoid sugar
intake. Adrenocorticotropic hormone (ACTH) and oral steroids (if any)
were tapered off two weeks before starting the dietary treatment. A
baseline video-electroencephalogram (EEG), whenever possible for a
minimum of 1 hour including at least one sleep-wake cycle was performed
in all children at the time of enrolment.
Eligible children were randomized using a
computer-generated random number list in two groups: mAD and LGIT. Both
groups were subjected to baseline one-week observation period, during
which parents were asked to maintain a daily seizure log. Anti-seizure
medications remained unchanged unless medically indicated, e.g. drug
toxicity, or status epilepticus, in which case appropriate changes were
made and the same was documented. Children were reviewed as outpatients
every two weeks during the trial period. A 24-hour dietary intake chart
was reviewed at each visit to compute calorie and carbohydrate intake
and to evaluate and reinforce compliance with the prescribed diet.
Weight was checked at each visit.
Percentage reduction in seizure frequency as compared
to the baseline was assessed as per the parental seizure records.
Seizure frequencies were recorded daily by parents. The seizure
frequency at 4 weeks and 12 weeks was calculated based on the average of
last one week. Based on comparison of these frequencies with baseline
one-week frequency, children were classified as seizure freedom, >50%
seizure reduction (50-90% reduction) and >90% seizure reduction. Parents
were asked to measure urine ketones twice weekly. EEG was repeated at 12
weeks. Tolerability of the diet and any adverse events was evaluated
using parental interviews at each visit, specifically asking about
vomiting, lethargy, poor appetite, refusal to feed and constipation, in
addition to others parental concerns. Liver and renal function tests and
fasting lipid profile were performed at baseline and repeated at the end
of 4 weeks and 12 weeks.
The sample size was estimated by use of null
hypothesis that the two groups would not have a significant difference
in seizure control outcome and by defining 30% as the minimum outcome
difference of clinical impor-tance. We estimated a sample size of 27 in
each group to enable detection of a difference that was significant at
5% with a power of 80%. Assuming 10% drop out, a sample size of 30 was
computed in each group.
Statistical analysis: Univariate analysis was
done to assess the distribution of data in groups and to choose the
appropriate statistical test. The proportion of children with seizure
freedom and greater than 50% and 90% seizure reduction were compared
between groups using Fisher exact test or Chi-Square test. The effect
size was expressed in terms of relative risk reduction (RRR) and 95%
confidence interval. For the purpose of RRR calculation, mAD group was
considered as intervention and LGIT group was considered as control;
achievement of seizure freedom, >90% reduction and >50% reduction were
considered as good outcome. An intention to treat analyses was
performed. A P value of less than 0.05 was considered
significant.
RESULTS
Of the 94 eligible participants, finally 30 children
received mAD and 30 received LGIT, of which five were lost to follow-up
at 12 weeks (Fig. 1). The baseline characteristics were
comparable the two groups. (Table I), except higher
proportion of Microcephaly among children in mAD group (P=0.03).
Table I Baseline Characteristics of Children With Drug Resistant Epilepsy (N=60)
|
Modified |
Low glycemic
|
| |
Atkins diet |
index treatment |
|
(n=30) |
(n=30) |
| Age (mo)a |
30(12,60) |
24(23.5,51) |
| Male gender |
22(73.3) |
25(83.3) |
| Age at onset of epilepsy (y)a |
0(0, 3) |
0.5(0, 3) |
| Type of seizure b |
|
|
|
Tonic clonic
|
14(46.7) |
19(63.3) |
| Epileptic spasms |
13(43.3) |
9(30) |
| Myoclonic |
0 |
2(6.7) |
| Focal |
2(6.7) |
0 |
| Neonatal problems |
21(70) |
17(56.7) |
| Birth asphyxia |
11(52.4) |
15(88.2) |
| Meningitis |
6(28.6) |
2(11.8) |
| Hyperbillirubinemia |
1(4.7) |
0 |
| Hypoglycemia |
3(14.2) |
0 |
| Microcephalyc |
16(53.3) |
7(23.3) |
| EEG findings |
|
|
| Multifocal epilepsy |
17(56.7) |
24(80) |
| Hypsarrhythmia |
11(36.7) |
6(20) |
| LGS |
2(6.7) |
0 |
|
EEG: Electroencephalography; LGS: Lennox-Gestaut syndrome; Data
in no. (%) except amedian (IQR); bFocal to bilateral tonic
clonic one child in modified Atkins diet group; All P>0.05
except cP=0.03. |
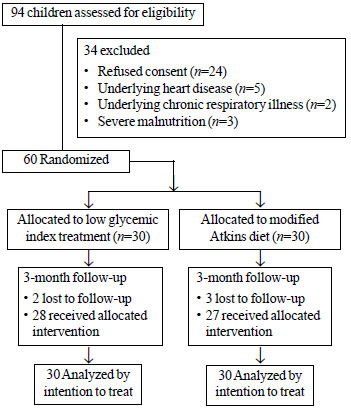 |
|
Fig.1 Study flow chart.
|
The proportion of children who achieved seizure
freedom at 12 weeks was comparable between the two groups (P=0.42),
and the chance of seizure freedom with mAD was better [RRR (95% CI) =
-1.5 (-10.9, 0.5)]. Similarly, the number of children who had more than
90% seizure reduction been also similar between the groups (P=0.21),
but the proportion of children with 50-90% seizure reduction was
significantly higher in LGIT group (P=0.03) at 12 weeks (Table
II). However, the significance of LGIT superiority at 12 weeks
needs to be interpreted in context of small effect size [RRR=0.4
(0.1-0.6)].
Table II Seizure Freedom and Adverse Effects Among Children With Drug Resistant Epilepsy
| Outcome measure |
Modified |
Low glycemic
|
RRR |
|
Atkins diet |
index treatment |
(95% CI) |
|
(n=30) |
(n=30) |
|
|
Seizure freedoma
|
| 12 wk |
5 (16.6) |
2 (6.6) |
-1.5 (-10.9-0.5) |
| 50-90% seizure reduction |
|
4 wkb |
19 (63.3) |
7 (23.3) |
-1.7 (-4.5, -0.3) |
|
12 wkc
|
13 (43.3) |
22 (73.3) |
0.4 (0.1,0.6) |
|
>90% seizure reductiona
|
| 12 wk |
9 (30) |
4 (13.3) |
-1.2 (-5.5, -0.2) |
| Adverse effects |
| Lethargy |
16 (53.3) |
20 (66.7) |
0.2 (-0.2, -0.5) |
| Constipation |
15 (50) |
9 (30) |
-0.6 (-2.2, -0.1) |
| Vomiting |
5 (16.7) |
3 (10) |
-0.7 (-5.4, -0.6) |
| Severe adverse effect |
2 (6.7) |
2 (6.7) |
0 (-5.6, -0.8) |
|
aNone of participants achieved seizure freedom or >90% seizure
reduction at 4 weeks. P<0.01; cP=0.03. RRR: Relative risk
reduction. |
The diet was well tolerated in both the groups.
Lethargy was the most common side effect. Two children in both groups
had significant weight loss as compared to baseline and severe
respiratory tract infections requiring hospitalization (serious adverse
event).
DISCUSSION
The present randomized control study with a limited
sample size shows that proportion of children with seizure freedom was
comparable between low glycemic index treatment and modified Atkins diet
for the treatment of drug-resistant epilepsy. LGIT diet was
significantly more effective in achieving >50% reduction in seizure as
compared to mAD diet at 12 weeks follow-up; although, with a small
effect size (RRR=0.4).
Around 47-56% of patients on LGIT are reported to had
achieved more than 50% reduction in seizure frequency [10-12]. In our
cohort, 73.3% achieved more than 50% reduction in LGIT group probably
because the previous studies were conducted among those with tuberous
sclerosis and adults. In a pediatric study from middle east, 78% of
children who received LGIT had achieved >50% reduction at the end of
2-month period [14]. The superiority of LGIT at 12 weeks in the present
study needs to be interpreted in the context of the small effect size.
LGIT in the present study had revealed disappointing results in terms of
early seizure control within four weeks, or achievement of seizure
freedom or >90% reduction. Most studies have used >50% reduction in
seizure frequency as their study outcome instead of seizure cessation
[10-12]. The other outcome measure includes percentage change in seizure
frequency [15]. Minimal adverse effect profile, good tolerability of
diet and efficacy in long term (3 months) are strong points to consider
LGIT as an alternative to mAD in achieving seizure reduction [14-15].
However, cessation of seizure in DRE looks unrealistic, and LGIT does
not promise to deliver the same.
Proportion of children with more than 50% reduction
dropped from 63.3% at 1-month to 43.3% at 3-month in the present study.
In contrast, previous studies have revealed slightly better efficacy
(52-68%) [6-9] of mAD at 3-month follow-up. Although reported compliance
with diet was satisfactory in the present study, it is difficult to
provide an alternative explanation for marginally reduced efficacy of
mAD and drop of efficacy from 1-month to 3-month follow-up. Studies have
demonstrated efficacy of mAD to a tune of 45.5% at 6-month follow up
[16]. We enrolled children with drug-resistant epilepsy and defined the
same as failure of two adequate and appropriate anti-seizure medication.
There has been lot of variation in the study inclusion in other studies.
Many have used terms like medically intractable epilepsy [10], and many
have adopted failure of three anti-seizure medication as their inclusion
criteria [14]. Most children in present study were in the age group of
30 months in both the study groups. This means that we had included
younger children with drug-resistant epilepsy. Many of them are either
West syndrome or those progressing to Lennox Gestaut syndrome as evident
from the type of seizure and their EEG findings. The adverse effect
profile and frequency was similar to previous report [7-9].
A recent Indian study [15] compared mAD, LGIT and KD
in a three-armed controlled trial. The study was conducted among 152
participants aged between 1-15 years with intractable epilepsy. They did
not find any significant difference in seizure reduction at 24 weeks in
the three groups. Nonetheless, patients on LGIT demonstrated >50%
seizure reduction with a better safety profile [15]. Authors had
considered percent seizure reduction as outcome measure limiting the
comparability of findings to present study, but both have demonstrated
comparable efficacy of mAD and LGIT.
Standardized definitions of drug-resistant epilepsy,
and outcome parameters including seizure freedom, and more than 50%
reduction in seizure were adopted to allow comparability of the results.
Limitations of the study include small sample size, relatively short
follow up period till three months, and lack of formal develop-mental
and cognitive assessment. In addition, serial EEGs were not performed in
the study to document improvement in the burden of epileptiform
discharges. We did not find a statistically significant difference
between mAD and LGIT in seizure freedom among children with drug
resistant epilepsy. Numerical superiority of LGIT over mAD at 12 weeks
for achieving >50% seizure reduction needs to be interpreted in the
context of limited sample size, short follow up period and small effect
size. Further multicenter randomized controlled trials may be considered
with larger sample size and longer follow-up period.
Note: Presented for V Balagopala Raju
Award at PEDICON 2020 in Indore, 9-12 January, 2020.
Contributors: SD, JSK: conceptualized the idea;
SG, JSK: drafted the manuscript; SD, JSK: provided intellectual inputs.
All the authors approved the final version of the manuscript.
Funding: None; Competing interests: None
stated.
|
WHAT IS ALREADY KNOWN?
•
Modified Atkins diet is an efficacious and less restrictive
alternative to ketogenic diet for management of drug-resistant
epilepsy.
WHAT THIS STUDY ADDS?
•
Proportion of children with seizure freedom was comparable
between low glycemic index treatment and modified Atkins diet
for the treatment of drug-resistant epilepsy.
|
REFERENCES
1. Kossoff EH, Zupec-Kania BA, Auvin S, et al.
Optimal clinical management of children receiving dietary therapies for
epilepsy: Updated recommendations of International Ketogenic Diet Study
Group. Epilepsia Open. 2018;3:175-92.
2. Sharma S, Jain P. The ketogenic diet and other
dietary treatments for refractory epilepsy in children. Ann Indian Acad
Neurol. 2014;17:253-8.
3. Nathan JK, Purandare AS, Parekh ZB, Manohar HV.
Ketogenic diet in Indian children with uncontrolled epilepsy. Indian
Pediatr. 2009;46:669-73.
4. Freeman JM, Vining EP, Pillas DJ, et al. The
efficacy of the ketogenic diet-1998: A prospective evaluation of
intervention in 150 children. Pediatrics. 1998;102:1358-63
5. Henderson CB, Filloux FM, Alder SC, et al.
Efficacy of the ketogenic diet as a treatment option for epilepsy:
Meta-analysis. J Child Neurol. 2006;21:193-8.
6. Sharma S, Jain P. The modified Atkins diet in
refractory epilepsy. Epilepsy Res Treat. 2014;2014:404202.
7. Tonekaboni SH, Mostaghimi P, Mirmiran P, et al.
Efficacy of the Atkins diet as therapy for intractable epilepsy in
children. Arch Iran Med. 2010;13:492-7
8. Kang HC, Lee HS, You SJ, et al. Use of a modified
Atkins diet in intractable childhood epilepsy. Epilepsia. 2007;48:
182-6.
9. Miranda MJ, Mortensen M, Povlsen JH, et al. Danish
study of a modified Atkins diet for medically intractable epilepsy in
children: Can we achieve the same results as with the classical
ketogenic diet. Seizure. 2011;20:151-5.
10. Pfeifer H, Thiele E. Low-glycemic-index
treatment: A liberalized ketogenic diet for treatment of intractable
epilepsy. Neurology. 2005;65:1810-2.
11. Kim SH, Kang HC, Lee EJ, et al. Glycemic index
treatment in patients with drug-resistant epilepsy. Brain Dev. 2017;
39:687-92.
12. Larson M, Anna P, Heidi TE. Low glycemic index
treat-ment for epilepsy in tuberous sclerosis complex. Epilep Res.
2011;99:180-2.
13. Kwan P, Arzimanoglou A, Berg AT, et
al. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad
Hoc Task Force of the ILAE commission on Therapeutic Strategies. Epilepsia.
2010;51:1069-77.
14. Karimzadeh P, Sedighi M, Beheshti M, et al. Low
glycemic index treatment in pediatric refractory epilepsy: the first
Middle East report. Seizure. 2014;23:570-2.
15. Sondhi V, Agarwala A, Pandey RM, et al. Efficacy
of ketogenic diet, modified atkins diet, and low glycemic index therapy
diet among children with drug-resistant epilepsy: A randomized clinical
trial. JAMA Pediatr. 2020;174:944-51.
16. Poorshiri B, Barzegar M, Tahmasebi S, et al. The
efficacy comparison of classic ketogenic diet and modified Atkins diet
in children with refractory epilepsy: A clinical trial. Acta Neurol
Belg. 2021:121:483-87.
|
|
|
 |
|

