|
|
|
Indian Pediatr 2020;57: 820-826 |
 |
Clinical Features and Outcome of SARS-CoV-2
Infection in Children: A Systematic Review and Meta-analysis
|
|
Jitendra Meena,1
Jaivinder Yadav,2
Lokesh Saini,2
Arushi Yadav3 and
Jogender Kumar2
From 1Departments of Pediatrics, All India
Institute of Medical Sciences, New Delhi; 2Post Graduate
Institute of Medical Education and Research, Chandigarh; and 3Department
of Radiodiagnosis, Government Medical College and Hospital, Chandigarh;
India.
Correspondence to: Dr Jogender Kumar, Assistant
Professor, Department of Pediatrics, Post Graduate Institute of Medical
Education and Research, Chandigarh, India.
Email: jogendrayadv@gmail.com
Published online: June 24, 2020;
PII: S097475591600203
|
|
Objective:
Knowledge about COVID-19 in children
is limited due to the paucity of reported data. The pediatric
age group comprises only less than 5% of total COVID-19
worldwide, therefore, large studies in this population are
unlikely in the immediate future. Hence, we planned to
synthesize the current data that will help in a better
understanding of COVID-19 in children.
Evidence acquisition:
Four different electronic databases (MEDLINE, EMBASE, Web of
Science, and CENTRAL) were searched for articles related to
COVID-19 in the pediatric population. We included studies
reporting disease characteristics and outcomes of COVID-19 in
patients aged less than 19 years. We performed a random-effect
meta-analysis to provide pooled estimates of various disease
characteristics.
Results: 27 studies (4857
patients) fulfilling the eligibility criteria were included in
this systematic review, from a total of 883 records. About half
of the patients had each of fever and cough, 11% (6-17%) had
fast breathing, and 6-13% had gastrointestinal manifestations.
Most of the patients had mild to moderate disease, and only 4%
had a severe or critical illness. Leukopenia was the commonest
reported laboratory abnormality.
Conclusion: Even among
the symptomatic COVID-19 cases, severe manifestations are seen
in very few children. Though fever and respiratory symptoms are
most common, many children also have gastrointestinal
manifestations.
Keywords: COVID-19, Gastrointestinal
symptoms, Mortality, Severity.
|
|
With the rapidly evolving severe acute respiratory
syndrome coronavirus-2 (SARS-Cov-2) pandemic, the knowledge about the
disease manifestations and severity has also evolved quickly. Due to its
resemblance to SARS, influenza, and other respiratory viruses, children
were initially thought to be more susceptible than adults. However, less
than 5% of total coronavirus disease (COVID-19) cases belong to the
pediatric age group, and the severity has been milder as compared to
adults [3,4]. Information regarding clinical manifestations and outcomes
of COVID-19 in adults is available due to a huge number of reported
cases, but the scenario for the pediatric population is different as our
knowledge about clinical and laboratory characteristics as well as
prognosis of COVID-19 is very limited.
Due to this difference in the manifestations of
COVID-19 among pediatric patients from adults, there is a need to
clarify the disease manifestations and course among children. We
performed this systematic review to synthesize the information on
clinical manifestations, laboratory findings, and outcome of COVID-19
among the pediatric population.
METHODS
We performed this systematic review to describe the
currently available literature on clinical features and outcomes of
COVID-19 in children between 1 month-19 years. Our primary aim was to
provide a pooled estimate of various clinical manifestations, disease
severity, and outcomes in children with SARS-CoV-2 infection.
This study was conducted following the meta-analysis
of observational studies in epidemiology (MOOSE) guidelines [5]. A
predefined search strategy was developed, and three investigators
independently performed a literature search in MEDLINE, EMBASE, Web of
Science, and CENTRAL (Cochrane central register of controlled trials)
for the original articles published between December 01, 2019, to May
10, 2020. The search strategy was targeted for patients aged less than
19 years with SARS-CoV-2 infection or COVID-19 and was based on two
basic groups of terminologies: study population
[pediatric / children / child / infant/adolescent], and terms related to
or describing COVID-19 and novel coronavirus (nCoV) infection. Terms
used for literature search were: COVID-19, coronavirus, SARS-CoV-2, 2019
nCoV, severe acute respiratory syndrome coronavirus 2, pediatric,
children, adolescent, and infant. Specific search strategies were
created for each search engine, by using the MeSH term and
above-described terms. The electronic search was also supplemented by a
hand search of bibliography of the included studies and relevant review
articles. We followed the Preferred Reporting Items for Systematic
Reviews and Meta-analyses (PRISMA) reporting guidelines [6]. No language
restrictions were used.
Study selection: A predefined set of the criteria
were used for study selection for this systematic review. Studies
enrolling children and clearly reporting data on their clinical features
were considered eligible for the review. Initially, two researchers
independently screened the title and abstract for the eligibility. Later
all the authors examined full-text articles for inclusion and exclusion
criteria. Studies were included if they met the following criteria: (i)
Patients aged less than 19 years with confirmed SARS-CoV-2 infection, (ii)
the study reporting clinical manifestations, disease severity, and/or
laboratory investigations and/or outcome of SARS-CoV-2 in children, and
(iii) all types of study design: cohort, cross-sectional studies,
case-control studies, and case series. Correspondences or letters
fulfilling the above criteria were also included. We excluded: (i)
case series reporting nine or fewer cases, (ii) studies reporting
only neonatal data, (iii) studies reporting about other serotypes of
coronavirus, (iv) narrative or systematic review, (v).
Conference proceedings, (vi) editorial, perspective, etc. not
meeting the inclusion criteria.
Data extraction and quality assessment: A
well-structured, standardized proforma was used for data extraction.
Four investigators independently extracted data from the full text of
the eligible studies. Extracted data included first author name, year,
country, journal, study design, study population information for risk of
bias assessment of the study, method, and type of sample used for
confirmation of SARS-CoV-2 infection, age and gender distribution of
cases, clinical and laboratory manifestations, major radiological
abnormalities, the severity of the disease, and reported outcomes in
studies. Any disagreement between two investigators was resolved through
discussion with another investigator. An independent researcher
rechecked the extracted data for its accuracy and completeness. Every
effort was made to avoid duplicity of data by screening full-text
articles of the included studies for author name, location, setting,
date and duration of the study, number of participants, and baseline
data. There is a possibility that the small case series/case report
might be a part of larger retrospective cohort. To address this issue,
we excluded case reports and small case series (up to nine patients).
The quality of the included studies in this systematic review was
assessed using the Newcastle Ottawa scale [7]. We used the following
scoring system: Good quality: 3 or 4 stars in selection domain, and 1 or
2 stars in comparability domain, and 2 or 3 stars in outcome/exposure
domain; fair quality: 2 stars in selection domain and 1 or 2 stars in
comparability domain, and 2 or 3 stars, in outcome/exposure domain; and
poor quality: 0 or 1 star in selection domain or 0 stars in
comparability domain or 1 star in outcome/exposure domain. Three
investigators independently assigned an overall risk of bias to each
eligible study, and if they disagreed, another researcher resolved the
discrepancy.
Data synthesis and statistical analysis: We
present the data with descriptive statistics and provide pooled
estimates of various parameters, wherever it was feasible to
meta-analyze the data. We categorized the disease severity into five
categories (asymptomatic, mild, moderate, severe, and critical) as
described by Dong, et al. [8]. We provid the pooled estimates of
various categories of disease severity from studies in which severity
classification was reported. Percentages and mean values were calculated
to describe categorical and continuous variables, respectively.
Meta-analyzed parameters were presented as pooled
estimates with a 95% confidence interval (CI). Meta-analysis was
performed using STATA version 14.2 (Stata Corp LLC, College Station,
Texas, USA).
We pooled data from individual studies using a random
effect model with the assumption that the frequency of clinical features
and other parameters will be variable across the studies. Heterogeneity
in studies was explored by inspection of forest plot as well as using
the chi-square test on Cochran’s Q statistics. Study heterogeneity was
assessed by using the Higgins and Thompson I2
method [9]. Publication bias was assessed by
Egger’s test.
RESULTS
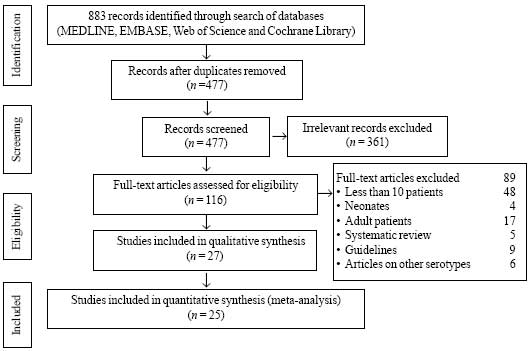
Using the above-described search strategy we found
883 articles, out of which 27 studies (4857 patients) (Fig. 1)
that met inclusion and exclusion criteria were included for final
qualitative synthesis, and 25 studies for quantitative synthesis (Web
Table I). A publication bias was found [bias (95% CI) = –2.9
(–4.5 to -1.3); P= 0.01].
 |
| |
Among 27 included studies, 21 were from China
[8,10-29], two each from Italy [30,31] and the Republic of Korea
[32,33], and one each from the United States of America [4], and Spain
[34]. Among the included studies, five were good, thirteen were fair,
and nine were of poor quality as assessed by the Newcastle Ottawa scale
[7]. A total of 4857 pediatric cases were reported in 27 eligible
studies. The mean (SD) age of the participants was 6.4 (3.4) years
(reported in eight studies) whereas the range varied from one month to
19 years. Twenty-three studies [8,10-25,27-32,34] (1777 patients)
reported separate data for gender; 1014 (57%) of all the patients were
male. Twenty-three studies [4,10-16,18-25,27-32,34] described the
frequency of specific symptoms in patients, of which only seventeen
reported disease severity.
Clinical manifestations and disease severity:
Sixteen studies reported data on the history of exposure to a SARS-CoV-2
infected patient and 91% (87-95%) of children had a history of contact
[10,11,14,15,17-25,27,28,30,34]. Among the included studies, only 17
studies reported data on asymptomatic SARS-CoV-2 infection
[8,11,12,15,16,19,21-25,27-32]. Almost one-fourth, 23% (17-30%) of
patients were asymptomatic. Since we included studies describing
clinical features, this number is not representative of the overall
proportion of asymptomatic cases in COVID-19 children. Twenty-three
studies (n-1330) reported data on specific symptoms
[4,10-16,18-25,27-32,34].
Fever was the commonest clinical feature and was seen
in almost half of the patients (Table I). Similarly, 45%
of symptomatic patients had cough, 11% had fast breathing, and 4-9% had
gastrointestinal manifestations.
Table I Clinical Features in Children with SARS-CoV-2 Infection
|
Symptoms |
Studies (patients)* |
Pooled estimates# |
Heterogeneity‡ |
P value |
|
Fever |
23 (1330) |
49 (41-58) |
90 |
<0.001 |
|
Cough |
23 (1330) |
45 (39-51) |
79 |
<0.001 |
|
Fast breathing |
10 (966) |
11 (6-17) |
87 |
<0.001 |
|
Coryza |
15 (1095) |
20 (13-26) |
87 |
<0.001 |
|
Sore throat |
15 (1012) |
14 (7-21) |
92 |
<0.001 |
|
Vomiting |
13 (1067) |
6 (4-9) |
48 |
0.03 |
|
Diarrhea |
15(1102) |
9 (6-13) |
80 |
<0.001 |
|
Abdominal pain |
7(604) |
4 (1-6) |
52 |
0.05 |
|
Myalgia |
6(418) |
10(1-18) |
83 |
<0.001 |
|
Headache |
6(546) |
10(1-19) |
92 |
<0.001 |
|
Hypoxia |
6(405) |
2 (1-3) |
0.0 |
0.84 |
|
*Number of studies (patients); #in % (95%) CI); ‡in I2%; P
value for I2. |
Of the all included studies, fifteen (n=1666)
provided specific information on disease severity
[8,11-13,15-20,22,24,25,30,31] (Table II). Most of these
patients had mild to moderate disease (96%), with a very small
proportion of patients having a severe manifestation (3%) like hypoxia,
dyspnea, and cyanosis (Table I). Only 1% of all the
symptomatic pediatric cases were critically sick (acute respiratory
distress syndrome, respiratory failure, shock, encephalopathy,
myocardial injury or heart failure, acute kidney injury etc).
Table II Severity of COVID-19 in Children
|
Disease
|
Studies
|
Pooled
|
Hetero- |
|
severity |
(patients)* |
estimates# |
geneity‡
|
|
Mild |
10 (1224) |
40(26-52) |
94 |
|
Moderate |
10 (1224) |
56(40-72) |
95 |
|
Severe |
19 (1677) |
3(1-5) |
68 |
|
Critically sick |
19 (1677) |
1(0.1-2) |
31 |
|
*Number of studies (patients); #in % (95%) CI); ‡in I2%; P
value for I2. |
To explore high heterogeneity, we did sensitivity and
subgroup (only for the quality of the studies) analysis and we did not
find any meaningful significant difference in the pooled estimates of
any of the clinical or laboratory feature. None except one study
provided data on relationship of severity of the illness with the age of
the patients. Dong, et al. [35] assessed the severity of illness
by age and reported that the young children, particularly infants are
more vulnerable to SARS-CoV-2 infection and had more severe disease.
Data on relationship of disease severity and gender was not reported in
children.
Table III Laboratory Findings in Children with COVID-19
|
Laboratory parameter* |
No. of studies
|
Pooled estimates
|
Heterogeneity |
P value for I2 |
|
(patients) |
% (95% CI) |
(I2%) |
|
|
Leukopenia |
17 (743) |
16 (11-22) |
81 |
<0.001 |
|
Leucocytosis |
17 (743) |
12 (7-17) |
71 |
<0.001 |
|
Lymphopenia |
19 (808) |
12 (8-17) |
75 |
<0.001 |
|
Elevated aspartate transaminase |
14 (665) |
15 (9-21) |
70 |
<0.001 |
|
Elevated alanine transaminase |
14 (665) |
10 (7-12) |
0.0 |
0.71 |
|
Elevated C-reactive protein |
13 (620) |
16 (10-22) |
79 |
<0.001 |
|
Elevated procalcitonin |
9 (476) |
25 (9-42) |
97 |
<0.001 |
|
Elevated erythrocyte sedimentation rate |
4 (125) |
9 (4-14) |
0.0 |
0.47 |
|
*The normal values of the laboratory parameters were as per
the authors of the given study. |
Laboratory and radiological abnormalities: Among
the laboratory findings (Table III), leukopenia was the
most commonly detected abnormality and was seen in almost one-fifth of
the patients. Twelve percent also had lymphopenia. On the other hand,
leucocytosis was detected in 12% (6-17%) patients. A significant
proportion (9-25%) of the cases had raised inflammatory markers
(erythrocyte sedimentation rate, C-reactive protein, and procalcitonin).
From the 15 studies which reported data on organ dysfunction, seven
patients had acute kidney injury and deranged liver function was
documented in 10-15% of patients [10-16,18,20-23,27,28,30]. Thirteen
studies reported data on radiological imaging (X-ray,
Computerized tomography scan), and the common radiological findings on
CT scan were ground-glass opacity (41%) and consolidation (16%)
[12,14-16,19-25,28,29,32].
Outcomes: Due to low event rate of outcome in
studies we did not meta-analyze this data. Mortality data was provided
in twenty-two studies (4476 patients) and overall, five deaths have been
reported [4,8,10-16,18-21,24-28,30,32-34] in these studies. Nineteen
studies [4,10-14,16,18-22,24,25,27,30-32,34] reported details on
hospital stay in 1670 children and of these 35 (2.1%) patients required
intensive care support for management and mechanical ventilator support
was needed for twelve patients (0.7%).
DISCUSSION
This review summarizes the clinical features,
laboratory findings, disease severity and prognosis from the available
studies about pediatric COVID-19 in the age group of one month to 19
years. The total number of reported pediatric cases is much less than
that reported in adults.
Similar to adults, the commonest clinical features
are fever and cough; however, their frequency is much lower in children
(60-100% in adults vs. 40-60% in children) [37,38]. On the other
hand, features like dyspnea, hypoxemia, and sputum production are more
frequently seen in adults. Like adults, the gastrointestinal
manifestations (diarrhoea, vomiting) are frequently seen in children and
sometimes may be the sole manifestation of COVID-19 [37-39]. Overall,
respiratory symptoms followed by gastrointestinal symptoms are the
predominant manifestations in children as well as adults [37,38]. These
findings play an important role in devising a screening strategy for
COVID-19 in children. The screening algorithms in adults rely primarily
upon fever and cough as these are present in more than 80% of the
patients; however, the same approach for the pediatric population is
likely to miss out 40-50% of the cases. Therefore, the screening
strategy for children should incorporate both respiratory and
gastrointestinal features.
Similar to adults, one-third of children with
COVID-19 children will have abnormal complete blood count [37,38].
Unlike other infections, leukopenia is more frequently encountered than
leukocytosis even in milder cases and should raise a high index of
suspicion for cases being evaluated for COVID-19. Around 15-25% of
pediatric cases may have raised C-reactive protein and procalcitonin in
alike other infections, and therefore inflammatory markers may not
differentiate COVID-19 from other infections. Serial procalcitonin
measurements in COVID-19 are shown to be useful in predicting disease
severity in adults, but similar data for the pediatric population is
lacking [40]. Like adults, 10-25% of pediatric COVID-19 cases may have
elevated liver enzymes (AST, ALT), however, its impact on disease
severity is unknown [41].
Most of the infections are asymptomatic in children
as well as adults and many of them are not reported. Therefore, we
confined ourselves to symptomatic cases only. Unlike other
influenza-like viral infections (MERS-CoV, H1N1) the children have less
severe COVID-19 affection as compared to adults [42]. Among the
symptomatic ones, mostly have a mild cough, cold, fever, and upper
respiratory tract infections only. The severe disease and the critical
disease (acute respiratory distress syndrome, respiratory failure,
shock, myocardial failure, and multiorgan dysfunction) are less frequent
in children (1-3%) as compared to adults (10-30%) [37,38-43]. Similarly,
the mortality associated with COVID-19 is much lower in the pediatric
population (less than 0.1 %) than that reported in adults (5-15%).
We excluded neonates to avoid clinical heterogeneity
as the clinical manifestations, mode of transmission, and outcome in the
neonatal population are quite different from the pediatric population
and are difficult to differentiate from the other neonatal illness. We
included studies published until May 10, 2020 and enrolled patients
tested positive for SARS-CoV-2 by RT-PCR. Also, it comprises information
from observational studies done in six different countries and is likely
to be the true representation of the clinical picture. The studies
included in previous reviews were exclusively from China and were
primarily case reports and small case series due to the non-availability
of larger studies at that time [36,44,45].
This study had several limitations. The pandemic is
still spreading and the available data are derived over a short
duration. The clinical features, laboratory abnormalities, and
radiological findings are limited to the studies describing symptomatic
patients admitted in the hospital. Therefore, it may not fully
characterize mild/asymptomatic patients not requiring hospitalization.
Also, we did not evaluate the impact of pre-existing comorbidities over
the clinical outcome; however, this is likely to be significantly less
than adults. As most of the studies are small, retrospective with lot of
heterogeneity and publication bias, the overall evidence (GRADE) is very
low. Therefore, the results should be interpreted cautiously.
Most of the children with COVID-19 were asymptomatic.
Amongst the symptomatic patients, the majority will have a mild
infection with very few requiring intensive unit care. Though fever and
other respiratory symptoms make up the commonest clinical presentation,
many may present with gastrointestinal symptoms. Therefore, a
comprehensive screening strategy including respiratory as well as
gastrointestinal features may be more useful.
Contributors: JM: conceptualized and designed the
study, formulated search strategy, collected data and analyzed the data,
and drafted the manuscript; JY: Acquisition and analysis of the data,
and critically revised the manuscript; LS: Acquisition and analysis of
the data, and critically revised the manuscript; AY: Supervised the
search strategy, data retrieval, and completeness of the data. Performed
hand search of the bibliographies and critically revised the manuscript;
JK: Conceptualized and designed the study, formulated search strategy,
collected and analyzed the data, and drafted the manuscript. All the
authors approved the final version of the manuscript and will be
accountable for all aspects of the work.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Most children with COVID-19 present with
mild symptoms, and carry good prognosis.
What This Study Adds?
• Though fever and cough are the most common
clinical presentation, many children may present with
gastrointestinal symptoms.
• A comprehensive screening strategy including respiratory as
well as gastrointestinal features (diarrhea and vomiting) may be
more useful.
|
REFERENCES
1. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al.
Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J
Med. 2020;382:1708-20.
2. World Health Organization. Coronavirus disease
(COVID-19) Situation Report- 129. Accessed as on May 29, 2020. Available
from:
https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200528-covid-19-sitrep-129.pdf?Sfvrsn=
5b154880_2.
3. Gupta N, Praharaj I, Bhatnagar T, Vivian Thangaraj
JW, Giri S, Chauhan H, et al. Severe acute respiratory illness
surveillance for coronavirus disease 2019, India, 2020. Indian J Med
Res. 2020;151:236–40.
4. CDC COVID-19 Response Team, Bialek S, Gierke R,
Hughes M, McNamara LA, et al. Coronavirus Disease 2019 in
Children — United States, February 12–April 2, 2020. MMWR Morb Mortal
Wkly Rep. 2020;69:422–6.
5. Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, et al. Meta-analysis Of Observational
Studies in Epidemiology (MOOSE) group. Meta-analysis of observational
studies in epidemiology: A proposal for reporting. JAMA.
2000;283:2008–12.
6. Moher D, Liberati A, Tetzlaff J, Altman DG, The
PRISMA Group. Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097.
7. Wells G, Shea B, O’Connell D, Peterson J, Welch V,
Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing
the quality of non-randomized studies in meta-analysis. Available from:
http://www.ohri.ca/programs/clinical_ epidemiology/oxford.asp.
Accessed May 28, 2020.
8. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et
al. Epidemiological characteristics of 2143 pediatric patients with
2019 coronavirus disease in China. Pediatrics. 2020;e20200702.
9. Higgins JPT, Thompson SG. Quantifying
heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
10. Chen J, Zhang Z-Z, Chen Y-K, Long Q-X, Tian W-G,
Deng H-J, et al. The clinical and immunological features of
pediatric COVID-19 patients in China [published online ahead of print,
2020 Apr 14]. Genes Dis. 2020;10.1016/j.gendis.2020.03.008.
11. Du W, Yu J, Wang H, Zhang X, Zhang S, Li Q, et
al. Clinical characteristics of COVID-19 in children compared with
adults in Shandong Province, China. Infection. 2020;48:445-52.
12. Ma H, Hu J, Tian J, Zhou X, Li H, Laws MT, et
al. A single-center, retrospective study of COVID-19 features in
children: A descriptive investigation. BMC Med. 2020;18:123.
13. Li H, Chen K, Liu M, Xu H, Xu Q. The profile of
peripheral blood lymphocyte subsets and serum cytokines in children with
2019 novel coronavirus pneumonia. J Infect. 2020;S0163-4453:30207-3.
[published online ahead of print, 2020].
14. Cai J, Xu J, Lin D, Yang Zhi, Xu L, Qu Z, et
al. A case series of children with 2019 novel coronavirus infection:
Clinical and epidemiological features [published online ahead of
print]. Clin Infect Dis. 2020;ciaa198.
15. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D.
Clinical and epidemiological features of 36 children with coronavirus
disease 2019 (COVID-19) in Zhejiang, China: An observational cohort
study. Lancet Infect Dis. 2020;20: 689-96.
16. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et
al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:
1663-65.
17. Shi Y, Wang X, Liu G, Zhu Q, Wang J, Yu H, et
al. A quickly, effectively screening process of novel corona virus
disease 2019 (COVID-19) in children in Shanghai, China. Ann Transl Med.
2020;8:241.
18. Zheng F, Liao C, Fan Q, Chen H, Zhao X, Xie Z,
et al. Clinical characteristics of children with coronavirus disease
2019 in Hubei, China. Curr Med Sci. 2020;40:275-80.
19. Song W, Li J, Zou N, Guan W, Pan J, Xu W.
Clinical features of pediatric patients with coronavirus disease
(COVID-19). J Clin Virol. 2020;127:104377.
20. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D.
Clinical and CT features in pediatric patients with COVID 19 infection:
Different points from adults. Pediatr Pulmonol. 2020;55:1169-74.
21. Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et
al. Characteristics of pediatric SARS-CoV-2 infection and potential
evidence for persistent fecal viral shedding. Nat Med. 2020;1-4.
22. Yaoling M, Shengying X, Min W, Simin Z, Wenhui D,
Qiong C. Clinical features of children with SARS-CoV-2 infection: An
analysis of 115 cases. Chinese J Contemp Pediatr. 2020;22:290-93.
23. Zhang B, Liu S, Zhang J, Xiao J, Zhu S, Dong Y,
et al. Children hospitalized for coronavirus disease 2019
(COVID-19): A multicenter retrospective descriptive study. J Infect.
2020;S0163-4453(20)30272-3. [published online ahead of print].
24. Xin T, Juan H, Fen Z, Zhou Y, Jieqiong L.
Clinical features of children with SARS-CoV-2 infection: An analysis of
13 cases from Changsha, China: Chinese J Contemp Pediatr.
2020; 22:294-98.
25. Wu Q, Xing Y, Shi L, Li W, Gao Y, Pan S, et al.
Co-infection and other clinical characteristics of COVID-19 in children.
Pediatrics. 2020;e20200961.
26. Shen KL, Yang YH. Diagnosis and treatment of 2019
novel coronavirus infection in children: A pressing issue. World J
Pediatr. 2020;1:3. [published online ahead of print].
27. Wang D, Ju XL, Xie F, Lu Y, Li FY, Huang HH,
et al. Clinical analysis of 31 cases of 2019 novel coronavirus
infection in children from six provinces (autonomous region) of northern
China. Chinese J Pediatr. 2020;58:269-74.
28. Tan Y, Tan B, Pan J, Wu J, Zeng S, Wei H.
Epidemiologic and clinical characteristics of 10 children with
coronavirus disease 2019 in Changsha, China. J Clin Virol.
2020;127:104353.
29. Li B, Shen J, Li L, Yu C. Radiographic and
clinical features of children with coronavirus disease (COVID-19)
Pneumonia. Indian Pediatr. 2020;57:423-26.
30. Parri N, Lenge M, Buonsenso D; Coronavirus
infection in pediatric emergency departments (CONFIDENCE) research
group. Children with Covid-19 in pediatric emergency departments in
Italy. N Engl J Med. 2020;NEJMc2007617.
31. Garazzino S, Montagnani C, Donà D, Meini A,
Felici E, Vergine G, et al. Multicentre Italian study of
SARS-CoV-2 infection in children and adolescents, preliminary data as at
10 April 2020. Euro Surveill. 2020;25:2000600.
32. Feng K, Yun YX, Wang XF, Yang GD, Zheng YJ, Lin
CM, et al. Analysis of CT features of 15 children with 2019 novel
coronavirus infection. Chinese J Pediatr. 2020;58:275-78.
33. COVID-19 National Emergency Response Center,
Epidemiology and Case Management Team, Korea Centers for Disease Control
and Prevention. Coronavirus Disease-19: The First 7,755 Cases in the
Republic of Korea. Osong Public Health Res Perspect. 2020;11:85-90.
34. Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia
FJ, Otheo E, Moraleda C, et al. Screening and Severity of
Coronavirus Disease 2019 (COVID-19) in Children in Madrid, Spain. JAMA
Pediatr. 2020;e201346. [published online ahead of print]
35. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et
al. Epidemiological characteristics of 2143 pediatric patients with
2019 coronavirus disease in China. Pediatrics. 2020;e20200702.
36. Ludvigsson JF. Systematic review of COVID-19 in
children shows milder cases and a better prognosis than adults. Acta
Paediatr. 2020;109:1088-95.
37. Rodriguez-Morales AJ, Cardona-Ospina JA,
Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging
features of COVID-19: A systematic review and meta-analysis. Travel Med
Infect Dis. 2020;34:101623.
38. Balla M, Merugu GP, Patel M, Koduri NM, Gayam V,
Adapa S, et al. COVID-19, modern pandemic: A systematic review
from front-line health care providers’ perspective. J Clin Med Res.
2020;12:215 229.
39. Tian Y, Rong L, Nian W, He Y. Review article:
Gastrointestinal features in COVID-19 and the possibility of faecal
transmission. Aliment Pharmacol Ther. 2020;51:843-51.
40. Lippi G, Plebani M. Procalcitonin in patients
with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin
Chim Acta. 2020;505:190-91.
41. Parohan M, Yaghoubi S, Seraj A. Liver injury is
associated with severe Coronavirus disease 2019 (COVID-19) infection: A
systematic review and meta-analysis of retrospective studies. Hepatol
Res. 2020;10.1111/hepr.13510. [published online ahead of print]
42. Zimmermann P, Curtis N. Coronavirus infections in
children including COVID-19: An overview of the epidemiology, clinical
features, diagnosis, treatment and prevention options in children.
Pediatr Infect Dis J. 2020;39:355-68.
43. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y,
et al. Epidemiological and clinical characteristics of 99 cases of
2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.
Lancet Lond Engl. 2020;395:507-13.
44. Castagnoli R, Votto M, Licari A, Brambilla I,
Bruno R, Perlini S, et al. Severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A
systematic review. JAMA Pediatr. 2020;10.1001/jamapediatrics.2020. 1467.
[published online ahead of print]
45. Chang TH, Wu JL, Chang LY. Clinical
characteristics and diagnostic challenges of pediatric COVID-19: A
systematic review and meta-analysis. J Formos Med Assoc.
2020;119:982-89.
|
|
|
 |
|

