|
|
|
Indian Pediatr 2020;57:801-804 |
 |
Pharmaceutical
Excipient Exposure in a Neonatal Intensive Care Unit
|
|
Sara Nasrollahi, 1
Neelathahalli Kasturirangan Meera1
and Sunil Boregowda2
From Department of 1Pharmacy Practice, Visveswarapura Institute of
Pharmaceutical Sciences, and 2Department of Pediatrics, KIMS Hospital
and Research Centre, Bangalore, India.
Correspondence to: Dr Sara Nasrollahi, Department of Pharmacy
Practice, Visveswarapura Institute of Pharmaceutical Sciences,
Bangalore, India.
Email: [email protected]
Received: August 20, 2019;
Initial review: December 30, 2019;
Accepted: April 22, 2020.
|
Objective: To study the excipients exposure among neonates in a
neonatal intensive care unit. Method: Prospective observational
study was conducted from January, 2017 to June, 2019. Details of
administered drugs were collected from the hospital case files. List of
excipients of formulations and their quantities were collected from
package insert leaflets or by contacting the manufacturers. Excipients
were grouped into four categories based on available safety data.
Calculated daily exposures to the excipients (mg/kg/day) were compared
with adult acceptable daily intake. Results: More than half of
the included 746 neonates were exposed to harmful excipients. 12.3% and
12.7% of neonates received higher than acceptable daily intake of sodium
metabisulphite and sunset yellow FCF, respectively. Conclusion:
There is a high risk of exposure of neonates to harmful excipients, and
clinicians need to be aware of this during neonatal care.
Keywords: Additives, Harm, Medications, Sodium metabisulphite.
|
|
E xcipients play a major
role in converting medicinal agents to acceptable dosage
forms[1]. Neonates are a vulnerable population and their drug
handling, pharmacokinetic and pharmacodynamic aspects are
different from older children. Neonates may be exposed to risks
and unwanted effects of excipients when they are administered
drug formulations. The reason could be immature physio-logical
functions leading to inadequate metabolism and excretion of such
excipients from body [2-4].
In the 1980s, ten neonatal gasping syndrome
and death were reported in a study as a result of toxicity of
benzyl alcohol (preservative) used in intravenous solutions [5].
Parabens, ethanol and propylene glycol are other examples of
excipients having harmful effects in neonates [1]. Therefore,
this study was conducted to assess types and amount of exposure
to excipients among neonates in neonatal intensive care unit
(NICU).
METHODS
This prospective observational study was
conducted in the NICU of a tertiary care teaching hospital in
Bangalore for duration of 2.5 years (January, 2017 to June,
2019) after approval from institutional human ethics committee.
Valid consent was given by the parents/guardians of included
study subjects who received at least one drug. Neonates dying
within 24 hours of birth were excluded. Demographic details
(gestational age, birthweight, gender, date of birth, post natal
age), length of stay, daily clinical progress of neonates,
information about prescribed medicines for all neonates
(indication, dose, frequency, route of administration, dosage
form and brand names) were recorded. Diagnoses were classified
according to ICD-10 (International statistical classification of
diseases and related health problems, 10th revision, 2016).
Administered drugs were classified according to WHO Anatomical
Therapeutic and Chemical (ATC) classification system.
Lists of excipients and their quantities
present in each prescribed formulation were collected by
referring to package insert leaflets (PIL) of drugs or
contacting the manufacturers. Excipients were categorized into
four groups as per Lass, et al. [1] viz., (a)
known to be harmful to neonates (adverse reactions reported in
neonates); (b) potentially harmful (adverse reactions
reported); (c) no safety data found (no data found in the
literature on human exposure and toxicity); and (d)
description of the excipient in PIL non-specific (description
does not allow a specific literature search).
Statistical analyses: Daily exposure to
excipients (mg/kg/day) were calculated based on available data
on quantity of excipients in formulations, and were compared
with acceptable daily intake (ADI) [6-8] for those which data of
ADI was available.
RESULTS
Of the 790 cases admitted to NICU during the
study period, 41 were excluded as they had received only
phototherapy (no medications), and 3 babies died within 24 hours
of birth. The baseline characteristics of 746 included neonates
are described in Table I. The most frequent
diagnoses were respiratory distress of newborn, neonatal sepsis
and congenital heart disease.
Table I Characteristics of Neonates (N=746)
|
Characteristics |
Value |
|
Male sex |
424 (56.8) |
|
Inborn babies |
576 (77.2) |
|
Gestational age, wk |
|
|
Term ( ³37) |
408 (54.7) |
|
Moderate to late preterm (32 to <37) |
274 (36.7) |
|
Very preterm (28 to <32) |
55 (7.4) |
|
Extremely preterm (<28) |
9 (1.2) |
|
*Length of stay, d |
|
|
Term |
5.7 (0.91) |
|
Moderate to late preterm |
8.4 (0.80) |
|
Very preterm |
19.27 (4.90) |
|
Extremely preterm |
27.33 (4.16) |
|
*Birthweight, g |
2480 (700) |
|
All values in n (%) except *mean (SD). |
The total number of prescribed drugs was
5535, and 77 different drugs were given. Systemic
anti-infectives, blood and blood forming organs, and alimentary
tract and metabolism class were the most commonly prescribed
classes of drugs. Intravenous (49, 63.6%) and oral (18, 23.3%)
were the most common routes of administration.
The qualitative and quantitative information
on excipients were available only for 35 and 15 drugs,
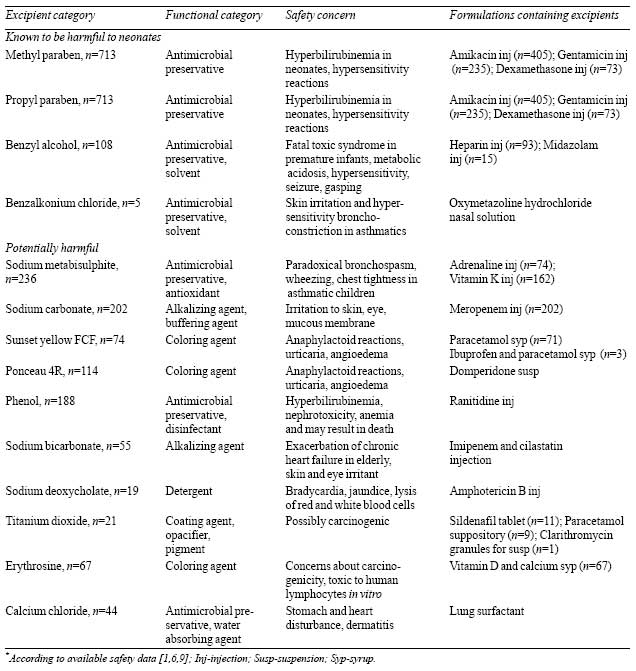
respectively. Total of 27 different excipients were identified.
Of all excipients, 4 (14.8%) and 10 (37%) were grouped under
category a and category b, respectively. These excipients were
present in 26% (20/77) of prescribed formulations, details of
which, including safety concerns [1,6,9], are given in
Table III. It was found that the highest proportion of
above mentioned excipient were present in systemic
anti-infectives.
Table II Amount of Exposure to Excipients in Neonates (N=746)*
|
Excipient |
Adult ADI [6-8] |
Daily dose exposure range |
Comparison with adult ADI |
|
Sodium metabisulphite |
0.7 mg/kg/d |
0.09-2.1 mg/kg/d |
‡Higher than ADI
|
|
Benzalkonium chloride |
0.1 mg/kg/d |
0.02-0.09 mg/kg/d |
Within ADI range |
|
Methyl paraben |
10 mg/kg/d |
0.03-1 mg/kg/d |
Within ADI range |
|
Propyl paraben |
10 mg/kg/d |
0.0003-0.09 mg/kg/d |
Within ADI range |
|
#Benzyl alcohol |
5 mg/kg/d |
0.016-1.3 mg/kg/d |
Within ADI range |
|
Phenol |
<50 mg in 10 h period |
0.1-0.8 mg in 12 h |
Within ADI range |
|
Sunset yellow FCF |
2.5 mg/kg/d |
0.3-4.2 mg/kg/d |
^Higher than ADI |
|
*Based on available data on quantity of excipients
present in drugs; ADI: Acceptable daily intake; #should
not be used in neonates; ‡12.3 % of exposures with use
of adrenaline injection; ^12.7 % of exposures with use
of paracetamol syrup. |
Emulsifier 472 C was the only identified
excipient of category c. Remaining excipients (8, 29.6%)
including yellow and red oxides of iron, caramel colour and
flavours were classified under category d. Daily exposure to
excipients of 8 injections (vitamin K, adrenaline, amikacin,
gentamicin, dexamethasone, heparin, midazolam and ranitidine),
oxymetazoline hydrochloride nasal solution and paracetamol syrup
were assessed and compared to ADI (table II).
|
Table III Classification of
Excipients to Which Neonates were Exposed*
|
 |
DISCUSSION
This study on qualitative and quantitative
excipient exposure among hospitalized neonates in India found
that 86.9% and 53.8% of neonates were exposed to at least one
excipient known to be harmful or potentially harmful,
respectively. Previous studies conducted in Brazil and Estonia
found that almost all neonates were prescribed drugs containing
at least one harmful excipient [1,3]. We found that harmful and
potentially harmful excipients were present in formulations that
were administered frequently and simultaneously. Hence neonates
could be at greater risk of toxic effects. Similarly, Fister,
et al. [10] reported that 51.6% of added excipients in
formulations were potentially harmful and harmful ones.
Coloring agents (ponceau 4R, sunset yellow
FCF, erythrosine and titanium dioxide) present in oral dosage
forms were included in potentially harmful category. Regulatory
status on colorants in different countries are not similar; in
the European union, medicines containing sunset yellow and
ponceau 4R must carry warning label concerning possible allergic
reactions [6]. In our study, Ponceau 4R was most frequently
observed colorant, though its use is banned in some countries
due to its effect on neurocognitive development and behavior
[2].
The use of category a or b excipients in
intravenous/oral formulations was much lesser in our study than
findings of Lass, et al. [1]. However, in another study
carried out in Spain [11], 32% of intravenous formulations and
62% of oral formulations contained at least one harmful
excipient. These differences can be explained by the
availability of information of excipients present in
formulations in different countries. We found high rates of
exposure (higher than ADI) to sodium metabisulphite and sunset
yellow FCF. Similar findings were reported by Akinmboni, et
al. [12] that 11% of neonates were exposed to a higher
amount of an excipient than the FDA/WHO recommended adult dose.
Daily exposure to phenol (in ranitidine
injection) was within the adult ADI range. However, literatures
show that use of ranitidine in very low birth weight neonates
can increase incidence of necrotizing enterocolitis, mortality
and infection [13]. Safety concern regarding use of ranitidine
was reported to the neonatologist. Daily exposure to benzyl
alcohol did not exceed the adult ADI. However, as per FDA
recommendation and label of the heparin injection, benzyl
alcohol containing formulations should not be used for neonates
and premature infants due to risk of fatal toxic syndrome in
neonates [6,14-16]. Unfortunately, 14.5% of neonates were
exposed to benzyl alcohol containing formulations. For 12.5% of
exposures, alternative preservative free formulation with the
same concentration of heparin sodium injection and similar cost
was available, which were suggested to the neonatologist.
Another study conducted in Netherlands showed that oral liquid
medicines without potentially harmful excipient were available
for 22% of medicines [17].
An important limitation of the present study
was the lack of data on neonatal ADI of excipients due to
barriers to conduct such studies. Another limitation was lack of
information about list of excipients and their quantities
present in each formulation. So, we could not assess the extent
of exposure to all excipients of formulations. However, our
findings with limited available data show that exposure to
harmful excipients is high, and awareness regarding their risks
needs to be raised.
Substitution of those medications with
excipient (harmful) free formulations, at least in high risk
conditions, will avoid unwanted risks. It is also important that
manufacturers disclose detailed qualitative and quantitative
information of excipients of formulations to clinicians and
clinical pharmacists, for risk/benefit assessment of selection
of drugs for neonates.
Ethical clearance: VIPS Human Ethics
Committee; IEC/2016-14 dated December 09, 2016.
Contributors: SN: collected the data,
analyzed the data and wrote the manuscript; NKM: designed,
monitored and supervised the study and approved the final
manuscript; SB: was the neonatologist who co-supervised the
study.
Funding: None; Competing interest:
None stated.
| |
|
What This Study Adds?
•
It is advisable that
excipient exposure be assessed while selecting medicinal
formulations for neonates.
|
REFERENCES
1. Lass J, Naelapää K, Shah U, Käär R,
Varendi H, Turner MA, et al. Hospitalized neonates in
Estonia commonly receive potentially harmful excipients. BMC
Pediatr:2012; 12:136.
2. Whittaker A, Currie AE, Turner MA, Field
DJ, Mulla H, Pandya HC. Toxic additives in medication for
preterm infants. Arch Dis Child Fetal Neonatal Ed: 2009;94:
F236-40.
3. Souza A Jr, Santos D, Fonseca S, Medeiros
M, Batista L, Turner M, et al. Toxic excipients in
medications for neonates in Brazil. Eur J Pediatr:
2014;173:935-45.
4. Kearns GL, Abdel-Rahman SM, Alander SW,
Blowey DL, Leeder JS, Kauffman RE. Developmental
pharmacology-Drug disposition, action, and therapy in infants
and children. N Engl J Med. 2003;349:1157-67.
5. Gershanik J, Boecler B, Ensley H,
McCloskey S, George W. The gasping syndrome and benzyl alcohol
poisoning. N Engl J Med.1982;307:1384-8.
6. Rowe RC, Sheskey PJ, Quinn ME. Handbook of
Pharmaceutical Excipients, 6th ed. Pharmaceutical press; 2009.
7. Commission of the European Communities.
Report from the Commission on Dietary Food Additive Intake in
the European Union. Available from:
https://ec.europa.eu/transparency/regdoc/rep/1/2001/EN/1-2001-542-EN-F1-1.Pdf.Accessed
January 10, 2019.
8. European food safety authority (EFSA).
Reasoned opinion on the dietary risk assessment for proposed
temporary maximum residue levels (MRLs) of
didecyldimethy-lammonium chloride (DDAC) and benzalkonium
chloride (BAC). Available from: https://efsa.onlinelibrary.
wiley. com/doi/epdf/10.2903/j.efsa.2014.3675. Accessed
January 13,2019.
9. European Medicines Agency.Committee for
Medicinal Products for Human Use (CHMP). Reflection Paper: for-mulations
of choice for the paediatric population. Available from:
https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paedi
atric-population_en.pdf. Accessed December 12, 2018.
10. Fister P, Urh S, Karner A, Krzan M,
Paro-Panjan D. The prevalence and pattern of pharmaceutical and
excipient exposure in a neonatal unit in Slovenia. J Matern
Fetal Neonatal Med. 2015;28:2053-61.
11. Garcia-Palop B, Movilla Polanco E, Cañete
Ramirez C, Cabañas Poy MJ. Harmful excipients in medicines for
neonates in Spain.Int J Clin Pharm. 2016;38:238-42.
12. Akinmboni TO, Davis NL, Falck AJ, Bearer
CF, Mooney SM. Excipient exposure in very low birth weight
preterm neonates. J Perinatol. 2018;38:169-74.
13. Terrin G, Passariello A, De Curtis M,
Manguso F, Salvia G, Lega L, et al. Ranitidine is
associated with infections, necrotizing enterocolitis, and fatal
outcome in newborns. Pediatrics: 2012;129:e40-5.
14. Committee on Fetus and Newborn, Committee
on Drugs. Benzyl Alcohol: Toxic Agent in Neonatal Units.
Pediatrics:1983;72:356-8.
15. Hiller JL, Benda GI, Rahatzad M, Allen
JR, Culver DH, Carlson CV, et al. Benzyl alcohol
toxicity: Impact on mortality and intraventricular hemorrhage
among very low birth weight infants. Pediatrics: 1986;77:500-6.
16. CDC. Neonatal deaths associated with use
of benzyl alcohol-United States. Available from:
https://www.cdc. gov/mmwr/preview/mmwrhtml/00001109.htm.
Accessed August 13, 2018.
17. Van Riet-Nales DA, de Jager KE, Schobben
AF, Egberts TC, Rademaker CM. The availability and
age-appropriateness of medicines authorized for children in the
Netherlands. Br J Clin Pharmacol:2011;72:465-73.
|
|
|
 |
|

