|
clinicopathological conference |
|
|
Indian Pediatr 2016;53: 815-821 |
 |
Recurrent
Ventricular Tachycardia and Peripheral Gangrene in a Young Child
|
|
*Kim Vaiphei, Pankaj C Vaidya, Pandiarajan Vignesh,
#Parag Barwad and Anju Gupta
From Departments of Pediatrics, *Histopathology and
#Cardiology, PGIMER, Chandigarh, India.
Correspondence to: Dr Pankaj C Vaidya, Associate
Professor, Department of Pediatrics, Advanced Pediatrics Centre (APC),
Postgraduate Institute of Medical Education and Research (PGIMER),
Chandigarh 160 012, India.
Email: [email protected]
Received: December 23, 2105;
Accepted: April 09, 2016.
Published online: July 01, 2016. PII:S097475591600017
|
A 10-year-old girl presented with sudden onset recurrent ventricular
tachycardia and symmetrical distal peripheral gangrene. She also had
pulmonary thromboembolism and cerebral sinus venous thrombosis.
Investigations revealed anemia, hemolysis, hypocomplementemia, and
elevated IgM anti-beta2 glycoprotein antibody levels. Electrocardiogram
and echocardiogram suggested features of a rare cardiac anomaly, which
was confirmed at autopsy.
Keywords: Antiphospholipid antibodies (APLA), Arrythmogenic
right ventricular dysplasia (ARVD), Thromboembolic manifestations,
Ventricular tachycardia.
|
|
Ventricular tachycardia in children is a
life-threatening emergency that needs prompt recognition and treatment.
When immediate correctable causes like dyselectrolytemia and drug
overdosages are not found, underlying structural defects of the heart
need to be looked for [1]. Thromboembolic manifestations in a child with
ventricular tachycardia are more likely to be due to underlying cardiac
pathology. We describe a child with ventricular tachycardia and
extensive thromboembolic features, where the final autopsy yielded a
rare cardiac anomaly. The etiology of thrombus was not only cardiac but
also an associated thrombophilic condition.
Clinical Protocol
History: A 10-years-old girl presented with
intermittent fever and cough of 12 days duration. She started developing
pain and bluish discoloration of fingers and toes on day 3 of illness
which progressed over the next few days to involve bilateral hands and
feet. She started developing difficulty in breathing on day 8 of
illness. This was associated with decreased urine output and generalized
swelling beginning from lower limbs and progressing to abdomen and face.
She had altered sensorium in the form of irritability and incoherent
speech for three days prior to presentation. Past history and family
history were unremarkable. She was a product of non-consanguineous
marriage. Her four other siblings were alive and healthy.
Examination: Her anthropometry was normal. At
admission, she was noted to have increased work of breathing with
tachypnea (respiratory rate 30/min), tachycardia (heart rate 170/min),
prolonged capillary refill and cold peripheries. All peripheral pulses
were palpable but feeble. Blood pressure in right arm was 90/68 mm Hg.
She had gangrene and edema of all fingers and toes extending to dorsum
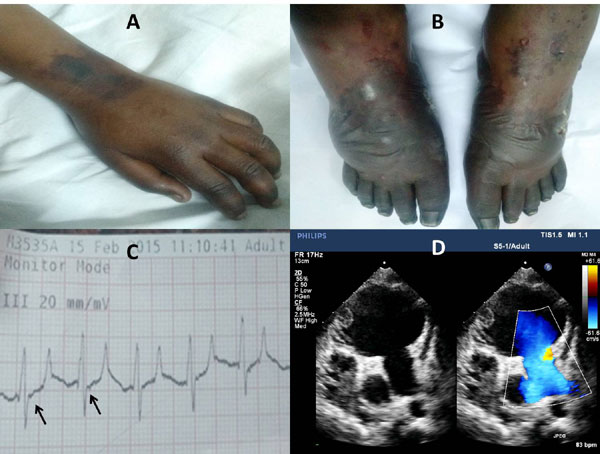
of hands and feet, respectively (Fig. 1a and 1b).
Pallor, periorbital puffiness and peripheral cyanosis were also noted.
Chest and cardiovascular examination revealed poorly localized apical
impulse, muffled heart sounds, S3 gallop and bilateral basal
crepitations. Soft, tender hepatomegaly with a span of 12 cm was also
found. She was conscious, oriented and obeyed commands but had
occasional aggressive behavior and incoherent speech. There were no
meningeal signs.
 |
|
Fig. 1 (a and b) Upper and
lower limbs of the index child showing bilateral symmetric
distal gangrene (c) Electrocardiogram lead III of the index
child showing epsilon waves and tall p waves (d) B mode
echocardiography with doppler - apical four chamber view showing
grossly dilated right ventricle (RV) with sacculations and a RV
clot.
|
Course and management: At admission, she was
started on nasal prong oxygen support, and inotropic support for shock.
Immediate cardioversion was done for electrocardiogram (ECG) -proven
ventricular tachycardia. Low molecular weight heparin (LMWH) infusion
was started for peripheral gangrene. Chest X-ray revealed
cardiomegaly and counts showed polymorphonuclear leucocytosis,
normocytic anemia and thrombocytopenia (Table I).
Peripheral smear was suggestive of hemolysis and reticulocyte count was
8.14%. Serum lactate dehydrogenase was elevated to 1707.6 U/L (Normal:
140-280 U/L). Complement C3 and C4 levels were low at admission. Lung
perfusion scintigraphy suggested intermediate probability of pulmonary
thromboembolism. Initial coagulogram showed prolonged prothrombin time
(PT) and activated partial thromboplastin time (aPTT) with low
fibrinogen and elevated d-dimer values. Considering her age, sex,
thrombocytopenia, hemolytic anemia and low complements at admission, a
possibility of systemic lupus erythematosus (SLE) with antiphos-pholipid
antibody syndrome (APS) was considered and she was given intravenous
methylprednisolone at a dose of 30 mg/kg/day for 3 days.
TABLE I Blood Counts, Biochemistry and Coagulogram of the Index Child
|
Investigations |
Day1 |
Day6 |
Day 12 |
Day16 |
Day 19 |
|
Hemoglobin (g/dL) |
10.1 |
10.8 |
8.9 |
8.1 |
10.5 |
|
White cell counts (/mm3) |
16,400 |
17,800 |
19,600 |
14,400 |
11,300 |
|
Differential counts* |
P76,L20 |
P90,L06 |
P84,L11 |
P88,L08 |
P86,L10 |
|
Platelets (/mm3) |
63,000 |
88,000 |
1,13,000 |
94,000 |
1,40,000 |
|
Serum sodium/potassium (mEq/L) |
130/5.4 |
141/2.8 |
140/5.6 |
138/4.1 |
134/4.1 |
|
Blood urea/creatinine (mg%) |
149/1.0 |
29/0.5 |
40/0.6 |
48/0.9 |
45/0.5 |
|
Serum total proteins/albumin (g/dL) |
- |
5.2/2.7 |
6.2/3.8 |
6.1/3.8 |
7.0/3.5 |
|
Serum total/conjugated bilirubin (mg/dL) |
2.05/1.01 |
0.99/0.43 |
1.23/0.46 |
1.25/0.36 |
0.7/- |
|
Serum AST/ALT (U/L) |
- |
188/195 |
57/101 |
58/96 |
34/72 |
|
Alkaline phosphatase (U/L) |
- |
169 |
159 |
165 |
146 |
|
Serum calcium/phosphorous (mg/dL) |
- |
7.6/2.3 |
8.4/3.3 |
7.8 /2.7 |
8.7/2.3 |
|
C-reactive protein (mg/dL) |
58.5 |
12.6 |
6.5 |
66.8 |
- |
|
Prothrombin time (s) |
40 |
33 |
23 |
19 |
15 |
|
International normalized ratio |
2.8 |
2.3 |
1.7 |
1.3 |
1.1 |
|
APTT (s) |
40 |
39 |
48 |
32 |
30 |
|
Fibrinogen (mg/dL) |
- |
0.90 |
- |
- |
4.8 |
|
*P: Polymorphonuclear leucocytes %, L: Lymphocytes %; AST:
Aspartate transaminase, ALT: Alanine transaminase, APTT:
Activated partial thromboplastin time. |
Extensive infection work-up done during hospital stay
was not contributory. Immunological work up revealed immune complex
vasculitis in skin biopsy and elevated IgM anti-beta2 glycoprotein
antibody levels. Other investigations including procoagulant work-up are
summarized in Table II. Protein C, protein S, antithrombin
III levels and lupus anticoagulant activity could not be done as the
child was already on LMWH. Contrast enhanced Magnetic resonance imaging
(MRI) of the brain showed ill-defined hyperintense foci in bilateral
cerebral hemisphere that showed diffusion restriction suggestive of
ischemic foci of acute or subacute nature. MR venography of brain
revealed features of chronic sinus venous thrombosis in parietal region.
Epsilon waves were detected in subsequent ECGs done during hospital stay
(Fig. 1c). Severely dilated right atrium and
ventricle with sacculations and aneurysms of the right ventricle (RV)
were noted in the echocardiogram suggesting a diagnosis of
arrhythmogenic right ventricular cardiomyopathy/ dysplasia (ARVC/D) (Fig.
1d). Clot was noted in RV with severe RV dysfunction. There
was no patent foramen ovale to explain systemic thrombo-embolic
tendency. There was no evidence of pulmonary arterial hypertension. On
day 9 of hospital stay, she developed recurrent episodes of ventricular
tachycardia needing repeated cardioversions and amiodarone infusion.
Permanent pacing or implantable defibrillator was planned and supportive
measures were continued. On day 20 of illness, she succumbed to
refractory ventricular tachycardia and cardiogenic shock.
TABLE II Investigations for Prothrombotic and Vasculitic Conditions in the Index Child
|
Investigations |
Results |
|
Radiology |
|
Lung perfusion scintigraphy |
Segmental wedge shaped mismatch defect in the right lower lobe–
intermediate probability of pulmonary thromboembolism |
|
Ultrasound doppler |
Normal flow demonstrated in both carotid, subclavian, axillary,
brachial, radial, renal, common femoral, popliteal, and anterior
tibial arteries. Flow could not be demonstrated in posterior
tibial arteries. |
|
CE-MRI* Brain with MRA* and MRV* |
MRV* showed obliterated straight sinus/proximal transverse
sinuses with venous collaterals in high parietal region ?
sequelae of chronic sinovenous thrombosis. MRA*- normal. |
|
Immunology |
|
Anti-nuclear antibody by IIF*, anti-double stranded DNA, anti-neutrophil
cytoplasmic antibodies by IIF*, anti-cardiolipin antibodies |
Negative |
|
Serum complements-C3/ C4 levels |
47/ 4 (Normal C3- 50 to 150 mg/dL, C4- 20 to 50 mg/dL) |
|
Anti- b2 glycoprotein 1 antibodies |
IgG- 2.8 U/mL, IgM- 11.6 U/mL (Normal <5 U/mL) |
|
Skin immunofluorescence |
IgM patchy band noted in blood vessels. IgG, IgA, C3 negative. |
|
Infectious diseases |
|
Blood cultures |
Sterile- repeated thrice |
|
Tuberculosis work up |
Negative (Tuberculin testing, gastric aspirates for AFB*
staining × 3) |
|
IgM Mycoplasma, IgM EBV VCA*, IgM CMV*, |
|
|
IgM anti-Hepatitis C, HIV* serologies |
Negative |
|
Hepatitis B surface antigen |
Negative |
|
Hematology |
|
Direct Coombs test |
Negative for C3d and IgG |
|
Peripheral smear for sickling, factor V Leiden mutation,
|
Negative |
|
flow cytometry based immuno-phenotyping for PNH* |
|
|
Serum homocysteine levels |
7.22 micromol/ L (Normal- 4.6 to 8.1) |
|
*CE-MRI: Contrast enhanced magnetic resonance imaging,
MRV-Magnetic resonance venography, MRA-Magnetic resonance
arteriography, IIF-Indirect immunofluorescence, AFB-Acid fast
bacilli, EBV VCA-Epstein Barr virus viral capsid antigen, CMV-
Cytomegalovirus, HIV-Human Immunodeficiency Virus,
PNH-Paroxysmal Nocturnal Hemoglobinuria. |
Unit’s final diagnosis: ARVC/D with RV failure,
RV clot and pulmonary embolism, ? Underlying prothrombotic condition, ?
Infection related or immune vasculitis, Disseminated intravascular
coagulation (DIC).
Clinical Discussion
The index child had features of cardiac involvement
(RV failure, ventricular tachycardia and shock) and thrombotic
manifestations at admission. In view of distinct right sided
cardiomyopathy with sacculations, ventricular tachycardia and epsilon
waves in ECG, diagnosis of ARVC/D appears likely [2-5]. Pulmonary
embolism and RV clots could be explained by right sided cardiac
pathology.
The index child had thrombotic manifestations both in
systemic and peripheral circulation- pulmonary embolus, symmetrical
peripheral gangrene and chronic sinovenous thrombosis on brain imaging.
She also had features of DIC- thrombocytopenia and prolonged PT and aPTT.
Recurrent ventricular tachycardia due to ARVC/D resulting in systemic
hypoperfusion and gangrene could explain symmetric peripheral gangrene.
However, it is impossible to explain preterminal febrile illness,
hemolysis, MRI evidence of chronic sinovenous thrombosis and low
complement levels with this diagnosis. Hence, thrombophilia (congenital
and acquired) is a strong possibility. Coming to the inherited
thrombophilias, serum homocysteine levels were normal and factor V
Leiden mutation was negative. Other inherited thrombophilias are protein
C deficiency, protein S deficiency, anti-thrombin deficiency,
dysfibrino-genemia, factor VIII mutations or prothrombin gene mutation,
for which the child could not be evaluated antemortem. The index child
had elevated IgM anti-beta2 GP1 antibody levels which may point to APS.
APS is an important acquired risk factor for both arterial and venous
thromboses. However, diagnosis of APS requires evidence of thrombosis
with elevated antibody titers (one of the three: lupus anticoagulant
(LA), anti-cardiolipin antibody (ACA), anti-beta2 GP1 antibody) on two
occasions at least 12 weeks apart. Diagnosis of catastrophic APS (CAPS)
requires demonstration of small vessel thrombotic manifestations of at
least three organs occurring within a week. APS is also known to cause
cardiomyopathy by causing multiple small vessel thrombosis and this is
one of the most important causes of death in APS [6,7].
Etiology of APS could be primary or secondary to
infections, autoimmune causes and malignancy. Extensive infectious
disease panel did not reveal any underlying infections. When we look at
autoimmune causes, the index child had two clinical criteria (hemolytic
anemia and thrombocytopenia) and two laboratory criteria (low
complements and elevated IgG anti-beta2 GP1 titres), which fulfils the
Systemic Lupus International Collaborating Clinics (SLICC 2012) criteria
for diagnosis of SLE. She also had evidence of immune complex mediated
vasculitis in skin biopsy. However, ANA and anti-dsDNA were negative,
making this diagnosis less likely.
Hypocomplementemia, immune complex mediated
vasculitis and embolic phenomenon can occur in infective endocarditis;
however, multiple blood cultures were sterile so this possibility does
not seem likely. The preterminal febrile illness with DIC, hemolysis and
low complement levels could be explained by an immune- or
infection-related vasculitis. So our final diagnosis would be
• Right sided cardiomyopathy ?ARVC/D ?APS related
• Pulmonary thromboembolism: related to RV
cardiomyopathy
• Systemic thrombotic manifestations ?APS related
Open Forum
Pediatric Rheumatologist: There is no going away
from the diagnosis of ARVC/D in this child as the index child had
refractory ventricular tachycardia and multiple sacculations and
dilatations of RV in echocardiogram. Histopathology of the heart may
show fibrofatty replacement of RV myocardium [2]. Though the clot in the
RV can explain the pulmonary embolism, the index child had features of
systemic microvascular/venous thrombosis, which prompted a procoagulant
work-up.
Adult Physician: The lesions in the extremities
are like purpura fulminans. The condition looks like CAPS and there may
be multiple thrombi in the cerebral, renal and extremity veins though
ARVC/D appears to be the primary heart disease.
Adult Rheumatologist: CAPS looks highly likely as
all the events in the index child occurred within a week.
Pediatric Rheumatologist 2: ARVC/D is distinctly
rare in pediatric population. Primary APS which caused the coronary
thrombosis and changes in RV appears more likely here.
Immunopathologist 1: Skin is the largest organ in the
body and looking at its biopsy helps in making diagnosis. We had a very
faint band test with occasional IgM deposits in the vessels suggestive
of immune complex vasculitis. I feel that APS related vasculitis is
likely in this child.
Pediatrician 1: Given the typical ECG and echo
findings, there is no doubt in the diagnosis of ARVC/D. This child
developed recurrent episodes of ventricular tachycardia which ultimately
took away the child. Pulmonary embolism can be explained by ARVC/D
itself; however to explain past sinovenous thrombosis and symmetric
peripheral gangrene with preserved pulses, prothrombotic state was
thought of. Preterminal illness could be infection related vasculitis.
Pediatrician 2: Index child fulfilled the SLICC
criteria for diagnosis of lupus. Yet, I think this child did not have
lupus as there was absence of ANA positivity by indirect immuno-fluorescence
testing on human cell lines. Though a question of semantics, strictly
speaking, we cannot use the term APS here because to call it as a
syndrome, we should repeat the antibody testing after 12 weeks [6].
Radiologist 1: What we found striking was the
enlarged veins in the posterior fossa that can be due to collaterals due
to chronic sinovenous thrombosis.
Adult physician 2: If cardiac MRI was done in
this child, it could have picked up the fibrofatty changes of the
myocardium in ARVD.
Cardiologist: We are dealing with two conditions:
procoagulant state and ARVC/D. Myocardial infarction related to right
coronary artery cannot cause such huge dilation of right atrium (RA) and
RV. Dilatation of RV secondary to pulmonary embolus is also less likely
as there was low pressure tricuspid regurgitation.
Adult Rheumatologist 2: This child had
symmetrical peripheral gangrene, low fibrinogen and prolonged PT and
aPTT. This looks more like DIC triggered by an infection which had a
focus in the heart like an infected clot.
Chairperson: There is no doubt that this child
had gangrene which was predominantly related to smaller vessels as the
peripheral pulses were palpable and this would bring in the possibility
of pro-coagulant disorders. Pathologist may clarify whether right heart
changes are related to the primary heart disease or it is secondarily
involved.
Pathology Protocol
A complete autopsy was carried out and the prosector
noted gangrene of both feet.
The heart weighed 208 g (normal range for this age
65-122 g) and was grossly enlarged. There was gross dilatation of the
right auricle, RA and RV with fresh auricular thrombus. RV anterior wall
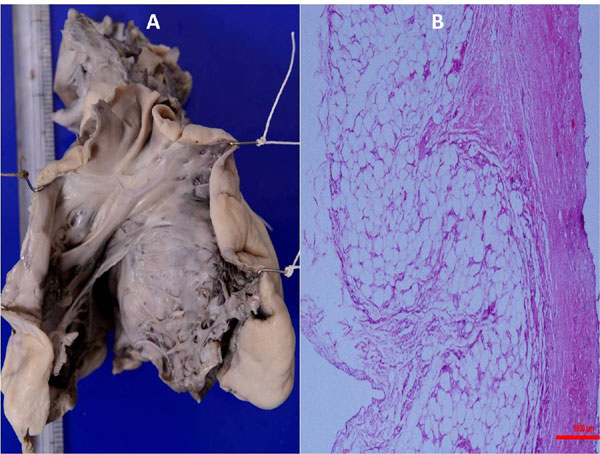
was papery thin with marked endocardial sclerosis (Fig. 2a).
Histology of the RV myocardium confirmed the gross thinning (just about
one mm) and replacement by collagenized tissue with dense
collagenisation of the endocardium with abundant elastic tissue
deposition and degeneration of cardiac myocytes (Fig. 2b).
Remaining RV myocardium was discoloured due to ischemic myonecrosis.
There were multiple mural thrombi both in RA and RV. Endocardium
overlying the interventricular septum along outflow tract of RV was
thickened and it also showed collagenisation. Pulmonary valve and artery
were dilated. Left atrium was of normal morphology. Left ventricle (LV);
however, was hypertrophied and discolored. Aortic valves, aorta and
coronaries were within normal limits. Histology of LV myocardium showed
diffuse subendocardial myocyto-lysis, sparse inflammatory cell
infiltration and focal myofibre disarray. Sections studied from the
atrio-ventricular (AV) nodal system showed total collagenisation. No
thrombus was identified in intra-abdominal or intra-thoracic vessels.
 |
|
Fig. 2 (a) Gross photograph of
the right side of the heart along the outflow tract showing
grossly thinned out right ventricular anterior wall and
endocardial sclerosis overlying the interventricular septum and
(b) Low power photomicrograph of the anterior right ventricular
wall showing totally fibrotic myocardium with no visible
myocardial fibres and thickened and elastisized endocardium. The
red coloured scale measure 1000 micron. (H&E, ×50).
|
Liver weighed 1032 g (normal range for this age
422-574 g) indicating gross enlargement. Capsular and cut surfaces of
the liver were grossly nodular and mottled due to bile staining. Normal
lobular architecture was lost and there was centro-central and
occasional centro-portal bridging fibrosis with many complete and
incomplete regenerative nodules. There was prominent centrizonal
sinuosoidal dilatation and congestion along with intra-sinusoidal
collagenisation. Larger sized portal tracts showed multiple dilated
intercommunicating portal venous channels. The scarred areas showed
deposition of excess hemosiderin pigment deposition.
Lungs showed evidences for aspiration and pulmonary
thromboembolism. Histology revealed pulmonary edema and fresh
intra-alveolar hemorrhages. Kidneys were swollen with marked medullary
congestion. Microscopic examination showed acute tubular necrosis and
granular, red blood cell and proteinaceous casts. Glomeruli and blood
vessels were within normal limits. There were multiple enlarged lymph
nodes in hilar, peri-pancreatic and mesentery regions measuring 10-15
mm. Sections showed reactive lymphoid follicles and sinus histiocytosis
with erythrophagocytosis. The spleen was of normal weight, and histology
showed depleted lymphoid follicle and evidence of extra-medullary
hematopoiesis in the sinusoids. The gastrointestinal tract (GIT) showed
mild mucosal congestion and histology showed changes of reflux
esophagitis and diffuse submucosal fibrosis along the entire length of
the intestine. The remaining organs were within normal limits.
Post-mortem bone marrow showed normal to mild hypercellularity with
presence of all the three hematopoietic cell lineages.
Final Autopsy Diagnosis
• Heart: ARVC/D associated with AV node fibrosis
and fresh mural thrombi of RA and RV
• Liver: Chronic passive venous congestion with
diffuse nodular regenerative hyperplasia
• Lungs: Pulmonary edema, thromboemboli and
aspiration.
• Kidneys: Acute tubular necrosis.
• GIT: Reflux esophagitis with diffuse
sub-mucosal fibrosis.
Open Forum
Immunopathologist 2: Most of the patients with
ANA negative lupus are positive for anti-Ro and anti-La, and even Hep2
cells can sometimes fail to detect these antibodies, sometimes
necessitating ELISA. These autoantibodies are known to cause heart
blocks and the pathology had shown fibrosed AV node. The index child
could have had this condition and the mother might be tested for those
antibodies.
Chairperson: It is beyond doubt that the child
had primary heart disease (ARVC/D). Distal gangrene cannot be explained
completely by the heart disease alone unless there was a communication
between right and left side, which has been negated by autopsy findings.
DIC precipitated by a different event or APS might have played part in
the presentation. The platelet counts never went below 80,000 cells/mm 3,
which is a little unusual for purpura fulminans.
Adult physician 1: Are the changes in ARVD due to
apoptosis or due to infiltration with inflammatory cells?
Pathologist: Apoptosis is part of the
degenerative process and especially in the pediatric age group, one
related condition - Uhl’s anomaly should be considered in the
differential diagnosis of RV failure and enlarged RV in echocardiogram.
However, the pathology is not suggestive of the same.
Discussion
ARVC/D is a cardiomyopathy characterized by
anatomical and functional abnormalities of predominantly RV. Genetic
origin is found in 30-50% individuals and is most commonly inherited as
autosomal dominant trait. Disease pathogenesis results from dysfunction
of desmosomes which are essential for electric conductivity and
contractility of myocardium. Most patients are men and it usually
presents between the ages of 20 and 40 [2]. In a large autopsy series of
ARVC/D, only 13% of cases were identified at less than 18 years [3].
Clinical phenotype evolves over a period of time. Common clinical
presentations are syncope, palpitations and ventricular tachycardia.
Arrhythmias are primarily a result of re-entry electrical circuits
generated from fibrofatty tissue in ARVC/D and can be triggered by
adrenergic stimulation such as exercise. Moreover, arrhythmias in ARVC/D
tend to be refractory to drugs [4]. Sudden death can be an initial
manifestation. In a large autopsy series of 1400 unexplained sudden
cardiac deaths, 200 (10.4%) cases had ARVC/D [3]. In the same series,
most cases of ARVC/D had abnormal Bundle of His and its branches because
of fat or fibrous tissue infiltration [3]. Disease progression in ARVD
might occur as periodic bursts rather than a continuous process. Disease
exacerbations can sometimes lead to life-threatening arrhythmias.
Definitive diagnosis of ARVC/D requires
histopathological demonstration of fibrofatty replacement of RV
myocardium. Expert consensus criteria requires either two major
criteria, one major plus two minor criteria or four minor criteria from
different groups for diagnosis (Table III) [4,5]. Index
child had histopathologically confirmed ARVC/D and many other features
of the consensus criteria.
Thromboembolic phenomena are observed in 4% of
patients with ARVD and it predominantly occurs in the right heart cavity
and pulmonary circulation [8]. Systemic thromboembolism in this
condition has been reported in only a few case reports [9,10].
Attenhoffer, et al. [9] reported a 36 year-old-woman with ARVC/D
and multiple large vein thromboses, where prothrombin gene mutation
G20210A was identified.
Anti-phospholipid antibodies (APLA) are identified in
4.2% of pediatric patients with thrombosis and it is the commonest
autoimmune etiology for acquired hyper-coagulable state [6].
Pathogenesis of thrombosis in APS is mainly due to activation of
platelets, monocytes and endothelium along with complement cascade
trigger by APLA. Around 50% of APS in children are associated with
autoimmune conditions, especially SLE [7]. Nearly 20% of patients with
primary APS can develop features of SLE in follow up. Presence of APS is
considered as a significant predictor for organ damage and mortality in
lupus patients [11]. Presence of LA correlates more with the
thromboembolic events in APS than the ACA or anti-beta2 GP1 assay.
Though anti-beta2 GP1 antibodies are more specific for thrombosis than
ACA, elevated titers of IgG anti-beta2GP1 antibody are much more
specific than elevated IgM which was observed in the index child [12].
Transient elevations of APLA are also found in association with various
infections and malignancies [13].
References
1. Pfammatter JP, Paul T. Idiopathic ventricular
tachycardia in infancy and childhood. J Am Coll Cardiol.
1999;33:2067-72.
2. Marcus F, Towbin JA. The mystery of arrhythmogenic
right ventricular dysplasia / cardiomyopathy. Circulation.
2006;114:1794-5.
3. Tabib A, Loire R, Chalabreysse L, Meyronnet D,
Miras A, Malicier D, et al. Circumstances of death and gross and
microscopic observations in a series of 200 cases of sudden death
associated with arrhythmogenic right ventricular cardiomyopathy and/or
dysplasia. Circulation. 2003;108: 3000-5.
4. Gemayel C, Pelliccia A, Thompson PD.
Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol.
2001;38:1773-81.
5. Basso C, Corrado D, Marcus FI, Nava A, Thiene G.
Arrhythmogenic right ventricular cardiomyopathy. Lancet.
2009;373:1289-300.
6. Ahluwalia J, Sreedharanunni S, Kumar N, Masih
J, Bose SK, Varma N, et al. Thrombotic primary antiphospholipid
syndrome: The profile of antibody positivity in patients from North
India. Int J Rheum Dis. 2014 Oct 8. [Epub ahead of print] doi: 10.1111/1756-185X.12479.
7. Avcin T, Cimaz R, Silverman ED, Cervera R,
Gattorno M, Garay S, et al. Pediatric antiphospholipid syndrome:
clinical and immunologic features of 121 patients in an international
registry. Pediatrics. 2008;122:e1100-07.
8. Wlodarska EK, Wozniak O, Konka M,
Rydlewska-Sadowska W, Biederman A, Hoffman P. Thromboembolic
complications in patients with arrhythmogenic right ventricular
dysplasia/cardiomyopathy. Europace. 2006;8: 596-600.
9. Attenhoffer Jost CH, Bombeli T, Schrimpf C,
Oechlin E, Kiowski W, Jenni R. Extensive thrombus formation in the right
ventricle due to a rare combination of arrhythmogenic right ventricular
cardiomyopathy and heterozygous prothrombin gene mutation G20210 A.
Cardiology. 2000;93:127-30.
10. Basso C, Thiene G, Corrado D, Angelini A, Nava A,
Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia,
dystrophy, or myocarditis? Circulation. 1996;94:983-91.
11. Descloux E, Durieu I, Cochat P, Vital-Durand D,
Ninet J, Fabien N, et al. Paediatric systemic lupus erythematosus:
prognostic impact of antiphospholipid antibodies. Rheumatology (Oxford).
2008;47;183-7.
12. De Groot PG, Urbanus RT. The significance of
autoantibodies against b2-glycoprotein
I. Blood. 2012;120:266-74.
13. Kratz C, Mauz-Körholz C, Kruck H, Körholz D, Göbel U. Detection
of antiphospholipid antibodies in children and adolescents. Pediatr
Hematol Oncol. 1998;15:325-32.
|
|
|
 |
|

