|
|
|
Indian Pediatr 2010;47: 757-760 |
 |
Diagnostic Re-evaluation of Children with
Congenital Hypothyroidism |
|
Priya S Nair, S Sobhakumar and Lalitha Kailas
From Department of Pediatrics, SAT Hospital, Medical
College, Thiruvananthapuram, India.
Correspondence to: Dr Priya S Nair, Department of
Pediatrics, Sree Gokulam Medical College,‘Anjali’, TC 2/564, Madathuvila
lane, Medical College PO, Trivandrum, Kerala 695 011, India.
Email: [email protected]
Received: March 18, 2009;
Initial review: April 27, 2009;
Accepted: October 8, 2009.
Published online:
2010 Jan 15.
PII:S097475590900172-1
|
|
Abstract
Objectives: To investigate the causes of
congenital hypothyroidism in children more than 3 years of age and to
document the frequency of transient vs permanent hypothyroidism.
Design: Hospital based observational study.
Setting: Pediatric endocrine clinic of a medical
college.
Patients: Children over 3 years of age, on
treatment for congenital hypothyroidism.
Intervention: Thyroid function test (TFT) and
thyroid ultrasound was done. Children with agenesis or hemiagenesis in
thyroid ultrasound were identified. In children with normal or equivocal
thyroid ultrasound, thyroxine was stopped and followed. Children with
abnormal TFT on follow up had thyroid scintigraphy with or without
potassium perchlorate discharge, after which, thyroid hormone supplement
was restarted. Children who remained euthyroid on follow up were labeled
as having transient hypothyroidism.
Main Outcome Measure: Proportion of children with
transient hypothyroidism.
Results: Among 36 children studied (20 boys and
16 girls), eighteen (50%) had transient hypothyroidism and fifteen
(41.7%) had thyroid agenesis. There was one with hemiagenesis, one with
ectopic thyroid and another with dyshormonogenesis (2.8% each). Initial
TSH level at the time of diagnosis was higher in permanent
hypothyroidism as compared with transient group (83.0 ± 31.6 vs 47.0 ±
33.1 mIU/mL; P= 0.002).
Conclusions: Thyroid hormone supplementation
could be discontinued in 50% of children diagnosed with congenital
hypothyroidism.
Key words: Children, Congenital hypothyroidism, Etiology,
India, Transient hypothyroidism.
|
|
T
he incidence of congenital
hypothyroidism in India varies from 1:2500 to 1:2800 live births(1).
Nearly 75% of all infants with congenital hypothyroidism have thyroid
dysgenesis, with hypoplasia or aplasia in 50 to 60%, ectopia in 25 to 35%
and dyshormonogenesis due to biosynthetic defects in 10 to 30%. Congenital
hypothyroidism could also result from transient abnormality in thyroid
gland function, which subsequently recovers. The possible explanations
include iodine deficiency, transplacental passage of maternal TSH-binding
inhibitory antibodies, and maternal exposure to radioiodine, iodine or
anti-thyroid drugs. In such situations, it may be possible to discontinue
thyroxine therapy. Trials of with-holding thyroxine therapy are reported
in the Western literature, but no such data is available from India. This
study was done to look at different causes of congenital hypothyroidism
and to determine the prevalence of transient hypothyroidism.
Methods
This hospital based observational study was conducted
from December 2005 to November 2006. Children over the age of 3 years
attending the pediatric endocrine clinic at SAT Hospital at Medical
College, Thiruvananthapuram and diag-nosed with congenital hypothyroidism
were included. Congenital hypothyroidism was defined as TSH more than 20
mIU/L at less than 2 weeks of age or TSH more than 10mIU/L after 2 weeks
of age(2). Exclusion criteria were unwillingness of parents or guardian to
give informed consent and severe illness (e.g. cardiac failure or chronic
CNS disorders) which could possibly be worsened by withdrawal of thyroid
hormone. All previous investigations such as ultrasound (US) or
radionuclide study, which were done to delineate the cause of congenital
hypothyroidism, were reviewed. Children who already had an imaging study
and were proven to have a form of permanent congenital hypothyroidism were
classified accordingly and excluded from further evaluation.
Blood was drawn for thyroid function tests (TFT) at
enrolment. A thyroid ultrasound was done. Children with agenesis or
hemiagenesis of thyroid on ultrasound were classified as such and excluded
from subsequent steps. In children with normal or equivocal US thyroid and
normal TFT, thyroxine was stopped. Parents were advised to monitor for
signs and symptoms of hypothyroidism. Four weeks after stopping thyroxine,
subjects were recalled for follow up. At that time, clinical assessment
for signs of hypothyroidism was done and blood was drawn for thyroid
function tests. Children with abnormal TFT underwent Tc-99m thyroid scan
or I-131 thyroid scan with or without potassium perchlorate discharge,
after which, thyroid hormone supplement was restarted at previous dose and
titrated for normal TFT.
Children with normal thyroid function at four weeks
were followed with serial TFTs at 8 weeks, 14 weeks and 6 months. If the
TFT remained normal, they were classified as transient hypothyroidism. If
TFT became abnormal in a subsequent follow up, they were investigated with
radionuclide scintigraphy. The protocol was approved by the Human Ethical
Committee of Medical College, Thiruvananthapuram.
Measurements for serum TSH and total T 4
were obtained at initial visit and at 4 wk; TSH at 8 wk and 14 wk, at the
Regional Cancer Center, Thiruvananthapuram, using chemiluminescence
immunoassay (reference range: TSH 0.25-6.3 mIU/L and T4 5.6-15 µg/dL).
Thyroid volume was calculated by ultra-sonography using the ellipsoidal
formula. Data from Gonzalez, et al.(3) were used to obtain cut-offs
for the lower limit of thyroid size. Normal thyroid volume was taken as
2.2 ± 1.3 mL for children aged 3-6 years, 3.0 ± 1.7 mL for 6-12 years and
5.7 ± 3.1 mL for adolescents. Agenesis was diagnosed if no thyroid tissue
was visualized in the neck. If only one lobe of thyroid was visualized
normally, while the other lobe was not seen, hemiagenesis was diagnosed.
When the thyroid volume appeared smaller than the lower limit of normal or
if the radiologist could not say a definite opinion, it was taken as
equivocal. Radionuclide tests were done at Regional Cancer Centre,
Thiruvananthapuram, if TSH increased on thyroxine withdrawal. I-131 was
the radionuclide agent used. Tc-99m study was done for one patient as the
probe for I-131 uptake study was not available at that time. Perchlorate
discharge test was done for one patient who had normal ultrasound and
I-131 scan, but became hypothyroid on thyroxine withdrawal.
Statistical analysis: Statistical analysis
was done using the SPSS for Windows statistical package (version 10.0.1).
Descriptive analysis and break up of the sample in different etiologic
categories was done. Hemiagenesis, ectopic and dyshormonogenesis groups
were clubbed together with agenesis and labeled as permanent
hypothyroidism. Differences between the permanent and transient
hypothyroidism groups were analyzed using t test.
Results
Thirty six children were included (Fig 1).
None of the patients had any documented proof of permanent congenital
hypothyroidism. All the patients were clinically euthyroid on thyroxine
replacement at the time of enrollment. The mean (± SD) age of the study
sample was 5.4 (± 2.7) years. There were 20 girls. The mean height centile
was 27.8 (range 3 rd to 90th centile).
Hypothyroidism was diagnosed and treatment started at a mean age of 3.8 (±
6.1) months (range newborn to 27 months) (61.1% in the neonatal period,
72.2% by 3 months, 86.1% by 6 months and 91.7% by 1 year of age).
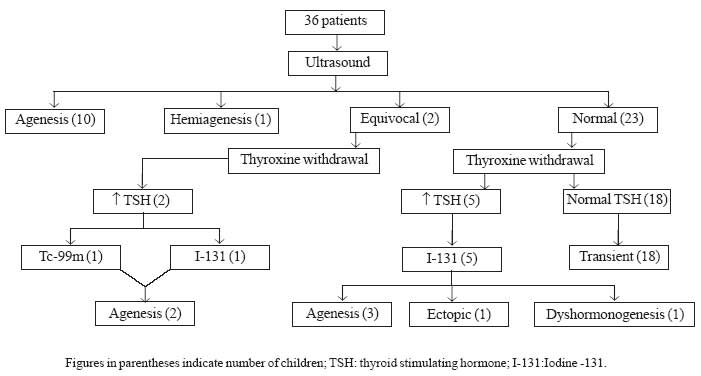 |
|
Fig.1 Patient flow with summary of results.
|
Transient hypothyroidism was seen in 18 patients (50%).
The other 50% had some form of permanent congenital hypothyroidism. Among
them, 15 patients (41.7%) had thyroid agenesis. There was one patient with
hemiagenesis, one with ectopic thyroid and another with dyshormonogenesis
(2.8% each). Initial TSH level at the time of diagnosis was significantly
higher in the permanent hypothyroidism group as compared with those with
transient hypothyroidism (83.0±31.6 vs 47.0±33.1 mIU/mL; P=0.002)
(Fig.2). There was significant difference in the thyroxine
dose between the two groups, when the total T 4
dose was considered. But when weight/kg body weight was considered,
although there was a trend towards higher dose requirement in the
permanent hypothyroidism group, the difference did not reach statistical
significance. There was no difference between the transient and permanent
hypothyroidism groups in the height centiles achieved.
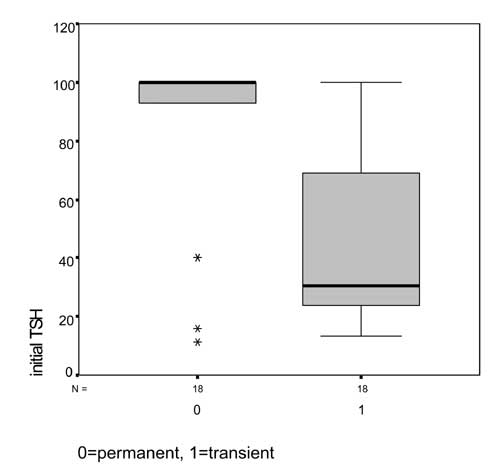 |
|
Fig.2 TSH at diagnosis (Box plot). |
Discussion
Among the 36 patients, 18 (50%) had transient
hypothyroidism. Previous studies in Indian children did not report any
incidence of transient hypothyroidism(4,5). This is probably due to later
age at diagnosis of hypothyroidism. In a study done by Eugster, et al.(6),
36% had transient hypothyroidism. Studies from other parts of the world
have reported transient hypothyroidism in 1-50% of children with
congenital hypothyroidism(7-11). TSH level at diagnosis was significantly
higher in children with permanent hypothyroidism. It may be of help to the
clinician while deciding to stop thyroxine therapy in a child diagnosed
with congenital hypothyroidism.
Our study was done in a relatively small sample of
children who were already diagnosed with congenital hypothyroidism and
were on follow up. Initial diagnostic details of these children were
obtained by retrospective chart review. This resulted in incomplete data
in some children. We do not have a congenital screening program, but most
of our patients were diagnosed early, probably due to higher awareness
among parents. We agree that unfortunately in many parts of the country,
congenital hypothyroidism is diagnosed late. In such a situation, the
diagnosis may be unequivocal and permanent. There may not be a need for a
trial of thyroxine withdrawal in such children.
We conclude that in children diagnosed with congenital
hypothyroidism, a standardized protocol of thyroxine withdrawal as
described above, after three years of age, is safe and will identify a
large proportion of patients in whom thyroxine could be safely withdrawn.
Acknowledgments
Dr N Roy, Department of Radiodiagnosis, Medical
College, Thiruvananthapuram and Dr Pradeep, Department of Nuclear
Medicine, Regional Cancer Center, Thiruvananthapuram for their help in
doing and analyzing the ultrasound and nuclear medicine scans in these
children. Dr PSN Menon, Head of Department of Pediatrics, Armed Forces
Hospital, Kuwait for advice regarding the study and the manuscript.
Contributors: Concept and design of the study was
provided by PSN, SS and LK, which was carried out by PSN and SS. Data
analysis and interpretation of results were done by PSN and SS and finally
revised and approved by LK. The final manuscript was approved by all
authors.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• There is a high prevalence of transient
hypothyroidism among children diagnosed with congenital
hypothyroidism in the West.
What This Study Adds?
• 50% of children in this study had transient
type of congenital hypothyroidism. A higher initial TSH level is
suggestive of permanent congenital hypothyroidism.
|
References
1. Desai MP. The thyroid gland. In: Desai MP,
Bhatia V, Menon PSN, editors. Pediatric Endocrine Disorders. 1st ed. New
Delhi: Orient Longman; 2001. p. 183-202.
2. Martin CR. Thyroid disorders. In: Cloherty
JP, Eichenwald EC, Stark AR, editors. Manual of Neonatal Care. 6 th
ed. Philadelphia: Lippincott, Williams and Wilkins; 2008. p. 19-27.
3. Gonzalez M, Gonzalez CP, Sanabria A.
Ultrasonographic estimation of the normal volume of the thyroid gland in
pediatric populations. Biomedica 2006; 26: 95-100.
4. Shankar SM, Menon PS, Karmarkar MG, Gopinath PG.
Dysgenesis of thyroid is the common type of childhood hypothyroidism in
environmentally iodine deficient areas of north India. Acta Paediatr 1994;
83: 1047-1051.
5. Desai MP. Disorders of thyroid gland in India.
Indian J Pediatr 1997; 64: 11-20.
6. Eugster EA, LeMay D, Zerin JM, Pescovitz OH.
Definitive diagnosis in children with congenital hypothyroidism. J Pediatr
2004; 144: 643-647.
7. Seeherunvong T, Churesigaew S. Etiologic study of
primary congenital hypothyroidism. J Med Assoc Thai 1998; 81: 653-657.
8. Weber GVM, Passoni A, Odoni M, Paesano PL, Dosio F,
Proverbio MC, et al. Congenital hypothyroidism with gland in situ:
diagnostic re-evaluation. J Endocrinol Invest 2005; 28: 516-522.
9. Davy T, Daneman D, Walfish PG, Ehrlich RM.
Congenital hypothyroidism. The effect of stopping treatment at 3 years of
age. Am J Dis Child 1985; 139:1028-1030.
10. Brown RS, Bellisario RL, Botero D, Fournier L,
Abrams CA, Cowger ML, et al. Incidence of transient congenital
hypothyroidism due to maternal thyrotropin receptor blocking antibodies in
over one million babies. J Clin Endocrinol Metab 1996; 81: 1147-1151.
11. Panoutsopoulos G, Mengreli C, Ilias I, Batsakis C,
Christakopoulou I. Scintigraphic evaluation of primary congenital
hypothyroidism: results of the Greek screening program. Eur J Nucl Med
2001; 28: 529-533.
|
|
|
 |
|

