|
|
|
Indian Pediatr 2020;57: 929-935 |
 |
Hyperinflammatory Syndrome in Children
Associated With COVID-19: Need for Awareness
|
|
Chandrika S Bhat, 1
Latika Gupta,2 S
Balasubramanian,3
Surjit Singh4 and
Athimalaipet V Ramanan5
From 1Pediatric Rheumatology Service,
Rainbow Children’s Hospital, Bangalore, Karnataka, India; 2Department
of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate
Institute of Medical Sciences, Lucknow, Uttar Pradesh India; 3Department
of Pediatrics, Kanchi Kamakoti CHILDS Trust Hospital, Chennai, Tamil
Nadu, India; 4Allergy Immunology Unit, Department of
Pediatrics, Advanced Pediatrics Centre, Post Graduate Institute of
Medical Education and Research, Chandigarh, India; and 5Bristol
Royal Hospital for Children and Translational Health Sciences,
University of Bristol, Bristol, UK.
Correspondence: Dr Chandrika S Bhat, Rainbow
Children’s Hospital, Marathahalli, Bengaluru 560 037, Karnataka, India.
Published online: July 15, 2020;
PII: S097475591600208
|
|
The pandemic of COVID-19
initially appeared to cause only a mild illness in children.
However, it is now apparent that a small percentage of children
can develop a hyperinflammatory syndrome labeled as Pediatric
inflammatory multisystem syndrome - temporally associated with
SARS-CoV-2 (PIMS-TS). Features of this newly recognized
condition may include persistent fever, evidence of
inflammation, and single or multi-organ dysfunction in the
absence of other known infections. Some of these children may
share features of Kawasaki disease, toxic shock syndrome or
cytokine storm syndrome. They can deteriorate rapidly and may
need intensive care support as well. The PCR test is more often
negative; although, most of the children have antibodies to
SARS-CoV-2. Although the pathogenesis is not clearly known,
immune-mediated injury has been implicated. We herein provide
current information on this condition, in order to raise
awareness amongst pediatricians.
Keywords: Kawasaki disease, Macrophage
activation syndrome, Multisystem inflammatory syndrome in
children and adolescents temporally related to COVID-19,
Pediatric inflammatory multisystem syndrome - temporally
associated with SARS-CoV-2 (PIMS-TS).
|
|
C
hildren younger than 18 years have been reported
to constitute only a small proportion of cases of coronavirus disease
(COVID-19). Whilst initial reports described an asympto-matic or milder
illness in children [1,2], several countries have now noticed a new
hyper-inflammatory syndrome affecting a small percentage of children
[3]. This condition appears to share features with pediatric
inflammatory diseases such as Kawasaki disease (KD) and Toxic shock
syndrome (TSS) [4].
The first case of classic KD with concurrent COVID-19
in a child was reported from United States [5]. Subsequently, health
authorities in the United Kingdom (UK) issued an alert describing a
serious illness requiring intensive care in children. A number of other
regions significantly affected by COVID-19 such as New York, Italy and
France also reported increasing numbers of children with a similar
inflammatory syndrome [3]; the first such case was reported from India
only recently [6]. The Royal College of Pediatrics and Child Health
(RCPCH) published a guidance to raise awareness amongst clinicians for
this newly recognized condition called Pediatric inflammatory
multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS)
[4]. A similar clinical entity was defined as the Multisystem
inflammatory syndrome in children and adolescents temporally related to
COVID-19 by the World Health Organization (WHO) [7] and Multisystem
inflammatory syndrome in children (MIS-C) associated with COVID-19 [8]
by Centers for Disease Control and prevention (CDC) (Box I).
Although little is known about the epidemiology, cases of PIMS-TS seem
to appear few weeks after the COVID-19 peak in the population. As of 13
May, 2020, there were more than 300 cases of suspected PIMS-TS in Europe
and North America [3]. With India lagging behind the peak curve, the
authors hypothesize that we may also see a spurt in this illness in the
coming days.
| |
|
Box I Proposed Case Definitions for the
Hyperinflammatory Syndrome Associated With COVID-19 [4,7,8]
World Health Organization
Children and adolescents 0-19 years of age
with fever >3 days AND two of the following:
(a) Rash or bilateral non-purulent
conjunctivitis or muco-cutaneous inflammation signs (oral, hands
or feet)
(b) Hypotension or shock
(c) Features of myocardial
dysfunction, pericarditis, valvulitis, or coronary abnormalities
(including ECHO findings or elevated Troponin/NT proBNP)
(d) Evidence of coagulopathy (by PT,
PTT, elevated D-dimer)
(e) Acute gastrointestinal problems (diarrhea,
vomiting, or abdominal pain)
AND
Elevated markers of inflammation such as ESR,
CRP or procalcitonin.
AND
No other obvious microbial cause of
inflammation, including bacterial sepsis, staphylococcal or
streptococcal shock syndromes.
AND
Evidence of COVID-19 (RT-PCR, antigen test or
serology positive), or likely contact with patients with
COVID-19.
Royal College of Pediatrics and Child Health
A child presenting with persistent fever,
inflammation (neutrophilia, elevated CRP and lymphopenia) and
evidence of single or multi-organ dysfunction.
This may include children fulfilling full or
partial criteria for Kawasaki disease.
Exclusion of any other microbial cause,
including bacterial sepsis, staphylococcal or streptococcal
shock syndromes, infections associated with myocarditis such as
enterovirus.
SARS-CoV-2 PCR testing may be positive or
negative.
Centers for Disease Control
An individual aged <21 years presenting with
fever, laboratory evidence of inflammation and evidence of
clinically severe illness requiring hospitalization, with
multisystem ( ³2)
organ involvement (cardiac, renal, respiratory, hematologic,
gastrointestinal, dermatologic or neurological)
(i) Fever ³38.0°C
for ³24
hours, or report of subjective fever lasting
³24
hours.
(ii) Laboratory evidence (but not
limited to) of one or more of the following: an elevated CRP,
ESR, fibrinogen, procalcitonin, D-dimer, ferritin, LDH, or
interleukin 6, elevated neutrophils, reduced lymphocytes and low
albumin.
AND
No alternative plausible diagnoses
AND
Positive for current or recent SARS-CoV-2
infection by RT-PCR, serology, or antigen test; Or COVID-19
exposure within 4 weeks prior to the onset of symptoms.
CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate;
LDH: Lactate dehydrogenase.
|
CLINICAL FEATURES
One of the initial reports [9] described a cluster of
eight children with hyperinflammatory shock. Mean age at presentation
was 8.8 years with a predilection for boys of Afro-Caribbean descent and
seven of these were above the 75 th
centile for weight. Mean duration of fever at presentation was 4.3 days.
Mucocutaneous changes (rash, conjunctivitis, peripheral edema) with
significant gastro-intestinal symptoms were noted in all of them. All 8
patients developed severe refractory shock with a mean ferritin level of
1086.6 ng/mL. One child required extra-corporeal membrane oxygenation
(ECMO) for refractory shock but eventually died after 6 days of
hospitalization. None of the children had respiratory symptoms and only
two tested positive for SARS-CoV-2 PCR, while all of them tested
positive for the antibody [9]. Ten children presenting with features of
classic or incomplete KD were reported from Italy [10] with mean age and
duration of fever of 7.5 years and 6 days, respectively. Apart from
gastrointestinal and mucocutaneous symptoms, menin-geal signs were also
reported in this subset. Half of them developed KD shock syndrome (KDSS)
with peak ferritin levels of 1176 ng/mL. In comparison to children with
KD in pre-pandemic times the current phenotype included older children
with more severe disease, significant cardiac involvement and macrophage
acti-vation syndrome (MAS) [10]. Again, only two tested positive for
SARS-CoV-2 PCR, but eight tested positive for the antibody. In both the
groups, inflammatory markers (C-reactive protein, procalcitonin,
ferritin, triglycerides, and D-dimer) were significantly elevated. An
abnormal echocardiogram with myocardial dysfunc-tion and coronary artery
abnormalities were observed in 60% children, and two also had coronary
aneurysms [10].
More recently, a French study [11] described a new
syndrome complex of acute heart failure and hyper-inflammation in
children. Initial presentation predomi-nantly included fever (100%) and
gastro-intestinal symptoms (80%) such as abdominal pain, vomiting and
diarrhea. Although mucocutaneous changes suggestive of KD were noted,
none of them met the criteria for classic KD. Echocardiography was
significant for left ventri-cular dysfunction with a low ejection
fraction. Inflam-matory markers (CRP, D-dimer) were raised in all.
Coronary artery dilatation was seen in 17%, but as opposed to classic
KD, none of them developed coronary aneurysms. Complete recovery was
seen in 71% of children, suggesting that myocardial edema rather than
necrosis was likely responsible for heart failure. This is in contrast
to the adult population, where myocardial necrosis has been incriminated
in the pathogenesis [11].
The importance of suspecting PIMS-TS in febrile
adolescent children with gastrointestinal symptoms during this pandemic
cannot be overemphasized. This unusual presentation was also reinforced
in a case series of eight children from UK, initially suspected to have
appendicitis [12]. Although they had very high CRP levels, abdominal
imaging demonstrated non-specific features (e.g. lymphadenopathy
or ileitis) rather than appendicitis. Subsequently, half of these
children required intensive care admission for hemodynamic instability.
Apart from peripheral or periorbital edema, none of them had features to
suggest classic KD and five tested positive for SARS-CoV-2 [12].
In a larger case series of 58 children (median age 9
years) from UK [13], all presented with fever and combi-nations of
abdominal pain (53%), diarrhea (52%) or rash (52%). Three clinical
patterns were identified in this cohort- fever with raised inflammatory
markers (39.6%) without features of KD, TSS or organ failure; shock
(50%) with evidence of left ventricular dysfunction (62%); and those
fulfilling criteria for KD. Coronary artery aneurysms were noted across
all three groups (8/58). Compared to other inflammatory disorders, those
with PIMS-TS were older and had lower hemoglobin levels and lymphocyte
counts, and higher white blood cell count, neutrophil count and CRP
levels (Table I) [13].
Table I Comparison of PIMS-TS With Classic KD, KDSS and TSS [13]
|
Features |
PIMS-TS (n=58) |
KD (n=1132) |
KDSS (n=45) |
TSS (n=46) |
|
Age at onset, y |
9.0 (5.7-14) |
2.7 (1.4-4.7) |
3.8 (0.2-18) |
7.38 (2.4-15.4) |
|
CRP, mg/L |
229 (156-338) |
67 (40-150) |
193 (83-237) |
201 (122-317) |
|
Hemoglobin, g/L |
92 (83-103) |
111 (105-119) |
107 (98-115) |
114 (98-130) |
|
Lymphocytes, ×109/L |
0.8 (0.5-1.5) |
2.8 (1.5-4.4) |
1.6 (1-2.5) |
0.63 (0.41-1.13) |
|
Ferritin, µg/L
|
610 (359-1280) |
200 (143-243) |
301 (228-337) |
– |
|
NT-Pro-BNP, pg/mL |
788 (174-10548) |
41 (12-102) |
396 (57-1520) |
– |
|
Troponin, ng/L |
45 (8-294) |
10 (10-20) |
10 (10-30) |
– |
|
D-dimer, ng/mL |
3578 (2085-8235) |
1650 (970-2660) |
2580 (1460-2990) |
|
|
Data are median (IQR); PIMS-TS: pediatric inflammatory
multisystem syndrome-temporally related to SARS-CoV-2, KD:
Kawasaki disease, KDSS: Kawasaki disease shock syndrome, TSS:
Toxic shock syndrome, CRP: C-reactive protein. |
It appears that these children may develop single or
multi-organ dysfunction with persistent fever and features of
inflammation (neutrophilia, elevated CRP and lymphopenia). This may
progress on to shock. In patients who turn out to be SARS-CoV-2 PCR
negative, other microbial causes need to be actively considered and
excluded [4]. In addition to KD and TSS, secondary hemophagocytic
lymphohistiocytosis (HLH) in associa-tion with common tropical
infections should also be considered in similar clinical settings. Based
on available data, we speculate that there could be three distinct
phenotypes of hyperinflammation in children (Table II).
PATHOGENESIS
Approximately two-thirds of patients with PIMS-TS are
COVID-19 PCR negative, a proportion of these being serologically
positive, suggesting an immune-mediated pathogenesis over a direct virus
invasion-mediated tissue injury. Infection with COVID-19 triggers the
formation of antibodies to viral surface epitopes. Virus neutrali-zation
is a direct function of the stochiometric concen-tration and affinity of
the antibodies. It is believed that low titer non-neutralizing
antibodies may accentuate virus triggered immune responses instead,
thereby increasing the risk of severe illness in affected individuals
[14]. While blocking antibodies against the angiotensin converting
enzyme (ACE) receptor binding regions (such as the RBD and HR2 region of
S protein) are deemed protective, those directed against nucleocapsid
and other epitopes on S protein are not [15,16]. Weak antibody coated
virus gets internalized by Fc receptors, followed by endosomal release
of the virion and subsequent Toll-like receptor and cytosolic RNA sensor
triggered IFN a
responses. These antibody dependent enhancement (ADE) responses have
been implicated in COVID-19 induced immune injury. Although evidence
base for this pathway is demonstrated for coronaviruses [16], the exact
role in PIMS-TS is only speculative [17].
MANAGEMENT
Conventionally, treatment of KD involves use of
intravenous immunoglobulin (IVIG) and high dose aspirin as first line
agents [18]. The use of IVIG for PIMS-TS may help in facilitating
neutralization of virus and associated superantigens and downregulation
of the inflammatory cytokines [19,20]. IVIG (2 g/kg) has been used in
most published series on PIMS-TS as first line therapy. The effects;
however, may be short-lived [9,10]. In those with features of classic
KD, it would be appropriate to consider use of aspirin (30-50 mg/kg/day
followed by 3-5 mg/kg/day) along with IVIG [18]. The role of aspirin in
children with hyperinflammation without features of KD is not known, and
we believe that it has a limited role in these children. Although the
role of anticoagulation is not clearly defined, it should be considered
on a case-by-case basis in children with hyperinflammatory syndrome. The
choice of anti-coagulation and their dosing regimen would also depend on
the presence of coronary aneurysms.
In select cases, especially those who do not respond
to IVIG, adjunctive immunomodulatory therapy may be necessary to control
inflammation. It is known that use of corticosteroids in KD is
associated with earlier resolution of fever and lower incidence of
coronary artery abnormalities [18,21]. Corticosteroids are also used as
first line therapy in children with MAS. On this basis, it is plausible
that these agents may be effective in PIMS-TS, especially in those with
features of cytokine release syndrome (CRS). Recently published case
series have shown that corticosteroids (initially pulse intravenous
methylprednisolone 10 mg/kg/day for 3 days followed by oral prednisolone
in a gradual tapering regimen) are useful adjuncts to IVIG in patients
with PIMS-TS [9,10,21].
Whilst not much is known about the pathogenesis of
PIMS-TS, it is clear that there is elevation of cytokines such as IL-1,
IL-6, IL-18 and IFN- a
in most children who develop MAS [22]. Although this does not
necessarily establish causality, specific cytokine blockade has resulted
in remission of MAS on many occasions [23]. Also, specific blockade of
TNF-a with
infliximab has been tried in children with KD resistant to IVIG [18].
Along with IL-6, several other cytokine blockade therapies are currently
under evaluation in adults with COVID-19. As we understand more about
targeted therapy in adults with COVID induced CRS, we might consider
trials of these agents in PIMS-TS [24,25]. Extrapolating these
data, it is possible that there may be a role for specific cytokine
blockade in PIMS-TS as well. Apart from one case report describing the
use of tocilizumab in a child with KD and SARS-CoV-2 [6], data on use of
biologics for this indication are still lacking. Until such data are
available, it would be reasonable to consider these therapies only under
special circumstances (in children with high CRP levels and those
refractory to IVIG/corticosteroids) either in controlled clinical trials
or by clinicians experienced in use of biologics. Where considered
appropriate, therapy with biologics such as tocilizumab (8 mg/kg) or
infliximab (5 mg/kg) should be considered. Based on existing evidence,
suggested management of children with SARS-CoV-2 related
hyperinflammation has been summarized in figure 1.
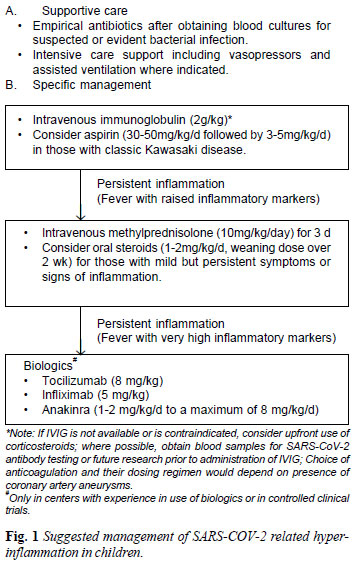 |
Apart from immunomodulation, supportive care plays a
key role in the management of these children. Deterioration can be
rapid, and it is important for clinic-ians to monitor for signs of
worsening inflammation [4].
FUTURE DIRECTIONS
The important answers lie in understanding the immune
origins of this condition. There is a need for clinical trials using
adaptive designs (Bayesian methodology) which would enable us to
evaluate therapies including IL-6, IL-1 and anti-TNF blockade in
children with this syndrome.
Despite the emerging literature, there are still a
lot of unknowns regarding SARS-CoV-2. It is important to gather data on
the condition to understand the damage caused and risk for recurrence as
well as long term implications including the risk for autoimmune disease
later in life. Real time surveillance studies such as the WHO clinical
data platform (https://apps.who.int/iris/handle/10665/332236) and
the British Pediatric Surveil-lance Unit (BPSU) study (https://www.rcpch.ac.uk/work-we-do/bpsu/study-multisystem-inflammatory-
syndrome-kawasaki-disease-toxic-shock-syndrome) can gather
information to help further our understanding of this disease. There is
now an overwhelming need for registries for data collection and
integration, especially in India [26,27]. Going forward, multicenter and
perhaps multi-national collaborative studies may be required to fill
existing gaps in our knowledge of the current pandemic and the new
syndrome in children.
In the Indian context, we perceive a definite need
for increased awareness of this unique clinical syndrome amongst parents
and pediatricians alike in the midst of multitude of several common
infections such as dengue, when a child presents with fever with
variable accompanying symptoms and signs and raised inflammatory
markers.
Contributors: CB, LG, AVR: substantial
contribution to the conception and design of the work, preparation and
finalization of the draft; CB, LG, SB, SS, AVR: substantial
contributions to the acquisition, analysis, and interpretation of data
for the work; SB, SS, AVR: Critical Revision for important intellectual
content; CB, LG, SB, SS, AVR: final approval of the version to be
published, and agreement to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Funding: None; Competing interests: None
stated.
REFERENCES
1. Balasubramanian S, Rao NM, Goenka A, Roderick M,
Ramanan AV. Coronavirus disease [COVID-19] in children - What we know so
far and what we do not. Indian Pediatr. 2020; 57(5):435-442.
2. Meena J, Yadav J, Saini L, Yadav A, Kumar J.
Clinical features and outcome of SARS-CoV-2 infection in children: A
systematic review and meta-analysis. Indian Pediatr.
2020;S097475591600203. [published online ahead of print, 2020 Jun 24].
3. European Centre for Disease Prevention and
Control. Pediatric inflammatory multisystem syndrome and SARS-CoV-2
infection in children – 15 May, 2020. ECDC: Stockholm; 2020.
4. Royal College of Pediatrics and Child Health.
Guidance–Pediatric multisystem inflammatory syndrome temporally
associated with COVID-19, 2020. Available from:
https://www.rcpch.ac.uk/resources/guidance-pediatric-multi
system-inflammatory-syndrome-temporally-associated-covid-19.
Accessed May 5, 2020.
5. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D,
Bradley SJ, et al. COVID-19 and Kawasaki disease: novel virus and
novel case. Hosp Pediatr. 2020 Jun; 10(6): 537-540. [E-pub ahead of
print].
6. Balasubramanian S, Nagendran TM, Ramachandran B,
Ramanan AV. Hyper-inflammatory syndrome in a child with COVID-19 treated
successfully with intravenous immunoglobulin and tocilizumab. Indian
Pediatr. 2020; S097475591600180. [E-pub ahead of print].
7. Multisystem inflammatory syndrome in children and
adolescents with COVID-19. 15 May 2020 Scientific brief: World Health
Organisation. Available from: https://www.
who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
Accessed May 31, 2020.
8. Centers for Disease Control and Prevention.
Emergency preparedness and response: Health alert network. Published May
14, 2020. Available from: https://emergency.cdc.gov/han/
2020/han00432.asp. Accessed May 22, 2020.
9. Riphagen S, Gomez X, Gonzalez-Martinez C,
Wilkinson N, Theocharis P. Hyperinflammatory shock in children during
COVID-19 pandemic. Lancet. 2020 May 23; 395 (10237):1607-1608.
10. Verdoni L, Mazza A, Gervasoni A, Martelli L,
Ruggeri M, Ciuffeda M, et al. An outbreak of severe Kawasaki-like
disease at the Italian epicentre of the SARS-CoV-2 epidemic: An
observational cohort study. Lancet. 2020; 395: 1771-78.
11. Belhadjer Z, Méot M, Bajolle F, Khraiche D,
Legendre A, Abakka S, et al. Acute heart failure in multisystem
inflammatory syndrome in children [MIS-C] in the context of global
SARS-CoV-2 pandemic. Circulation. 2020 May 17;
CIRCULATIONAHA.120.048360. [E-pub ahead for print].
12. Tullie L, Ford K, Bisharat M, Watson T, Thakkar
H, Mullassery D, et al. Gastrointestinal features in children
with COVID-19: an observation of varied presentation in eight children.
Lancet Child Adolesc Health. 2020;4: e19-e20.
13. Whittaker E, Bamford A, Kenny J, et al.
Clinical characteristics of 58 children with a pediatric inflammatory
multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;
e2010369. [E-pub ahead for print].
14. Yu H, Sun B, Fang Z, Zhao J, Liu X, Li Y, et
al. Distinct features of SARS-CoV-2-specific IgA response in
COVID-19 patients. European Resp J. 2020; 2001526. [E-pub ahead for
print].
15. Iwasaki A, Yang Y. The potential danger of
suboptimal antibody responses in COVID-19. Nature Reviews Immunology.
2020. Available from: http://www.nature.
com/articles/s41577-020-0321-6. Accessed May 29, 2020.
16. Wang Q, Zhang L, Kuwahara K, Li L, Liu Z, Li T,
et al. Immunodominant SARS coronavirus epitopes in humans
elicited both enhancing and neutralizing effects on infection in
non-human primates. ACS Infect Dis. 2016; 2(5):361-376.
17. Shen L, Fanger MW. Secretory IgA antibodies
synergize with IgG in promoting ADCC by human polymor-phonuclear cells,
monocytes, and lymphocytes. Cellular Immunology. 1981; 59(1):75-81.
18. McCrindle BW, Rowley AH, Newburger JW, Burns JC,
Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term
management of Kawasaki disease: A scientific statement for health
professionals from the American Heart Association. Circulation.
2017;135: e927-e99.
19. Burns JC, Franco A. The immunomodulatory effects
of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev
Clin Immunol. 2015;11:819-25.
20. Lo MS, Newburger JW. Role of intravenous
immunoglobulin in the treatment of Kawasaki disease. Int J Rheum Dis.
2018;21:64-9.
21. Kobayashi T, Saji T, Otani T, Takeuchi K,
Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus
prednisolone for prevention of coronary artery abnormalities in severe
Kawasaki disease [RAISE study]: A randomised, open-label,
blinded-endpoints trial. Lancet. 2012; 379:1613-20.
22. Schulert GS, Grom AA. Pathogenesis of macrophage
activation syndrome and potential for cytokine- directed therapies. Annu
Rev Med. 2015; 66:145-59.
23. Schulert GS, Grom AA. Macrophage activation
syndrome and cytokine-directed therapies. Best Pract Res Clin Rheumatol.
2014; 28:277-92.
24. Mehta P, McAuley DF, Brown M, Sanchez E,
Tattersall RS, Manson JJ, et al. COVID 19: Consider cytokine
storm syndromes and immunosuppression. Lancet. 2020; 395:1033-34.
25. Pacha O, Sallman MA, Evans SE. COVID-19: A case
for inhibiting IL-17? Nat Rev Immunol. 2020; 20:345-46.
26. Acharyya BC, Acharyya S, Das D. Novel coronavirus
mimicking kawasaki disease in an infant. Indian Pediatr. 2020;
S097475591600184 [published online ahead of print, 2020 May 22].
27. Rauf A, Vijayan A, John ST, Hrishnan R, Latheef
A. Multisystem inflammatory syndrome with features of atypical Kawasaki
disease during COVID-19 pandemic. Indian J Pediatr. 2020;
S12098020033571 [published online ahead of print, 2020 May 28].
|
|
|
 |
|

