|
|
|
Indian Pediatr 2020;57: 899-903 |
 |
WHO 2009 Warning Signs as Predictors of Time
Taken for Progression to Severe Dengue in Children
|
Priya Sreenivasan, 1,2 S
Geetha1,2 and A
Santhosh Kumar1
From Department of 1Pediatrics, and 2Clinical
Epidemiology Resource and Training Centre (CERTC),
Government Medical College, Thiruvananthapuram, Kerala.
Correspondence to: Dr Priya Sreenivasan, Associate
Professor of Pediatrics, Government Medical College,
Thiruvananthapuram, Kerala, India.
Email:
[email protected]
Received: August 14, 2019;
Initial reviews: November 14, 2019;
Accepted: June 10, 2020.
|
Objective: To identify WHO 2009
warning signs that can predict time taken for progression to
severe dengue in a pediatric population.
Design: Prospective analytical study
over 1 year and 2 months.
Setting: Tertiary care center.
Participants: 350 children aged 1
mo-12 y with serologically confirmed dengue without
co-morbidities/co-infections; conse-cutive sampling.
Procedure: At admission, clinical and
laboratory details were noted. Disease progression, time of
onset of each warning sign, hematocrit, and platelet counts
were recorded daily till discharge/ death. If progressing to
severe dengue, its time of onset was noted. Time to event
analysis with Log Rank test, Kaplan Meier plots and Cox
Proportional Hazards Model was done.
Outcome Measures: Primary
outcome was time interval from onset of first warning sign
to onset of severe dengue (defined as per WHO 2009
guidelines). Predictors were WHO 2009 warning signs:
abdominal pain, lethargy, persistent vomiting, mucosal
bleed, clinical fluid accumulation, hepatomegaly >2 cm,
hematocrit ³0.40
and platelet count <100x109/L.
Results: Among 350 children followed
up completely till discharge/death, 90 developed severe
dengue (event) while 260 did not (censored). Median age of
study population was 7.75 y. Clinical fluid accumulation [(P=0.002,
Hazard Ratio (HR) 2.19, 95% CI 1.33-3.60)] and hematocrit
³0.40
[(P=0.009, HR (95%CI) 1.715, (1.13-2.60)] were
significant in univariate analysis. Final multivariate model
includes clinical fluid accumulation [(P=0.02, HR
(95%CI) 1.89, (1.116-3.202)], hematocrit
³0.40
(P=0.07), mucosal bleed (P=0.56) and
persistent vomiting (P=0.32).
Conclusion: WHO warning signs that
predict time taken for progression to severe dengue in
children include clinical fluid accumulation, hematocrit
³0.40,
persistent vomiting and mucosal bleed. Study results have
implications in policy making and practice guidelines to
triage children attending a health care facility with or
without warning signs.
Keywords: Hematocrit, Management, Outcome,
Prognosis.
|
D
engue is a globally prevalent
arboviral infection with high morbidity and mortality in
India [1]. Kerala reported 19,912 dengue cases with 37
deaths in 2017 [2]. Dengue is dynamic with febrile phase,
critical phase (appearance of warning signs at/around
defervescence mark onset of capillary leak) and convalescent
phase [3]. Seven warning signs viz. abdominal pain,
lethargy, mucosal bleed, persistent vomiting, clinical fluid
accumulation, hepatomegaly >2 cm and rising hematocrit with
a concurrent fall in platelet count below 100×109/L
are evidence-based signs selected by the World Health
Organization (WHO) [3,4]. Potentially lethal severe dengue
can manifest as shock, severe bleed or severe organ
impairment in the critical phase or in the febrile phase
without preceding warning signs [3]. Close monitoring and
timely initiation of intravenous fluids in the presence of
any warning signs remain the only effective treatment
modality in dengue [3]. Severe dengue manifests as mostly
shock in children and as severe bleeding and organ
impairment in adults [5].
A prognostic prediction model using seven
WHO warning signs to determine severe dengue in children has
been published earlier [6]. Dynamicity of illness can be
captured by taking into consideration the time to time
variations in clinical and laboratory variables [7]. The
present study aimed to identify warning signs which can
predict time taken for progression to severe dengue in
children admitted to a tertiary care center.
METHODS
This prospective study was done in a
tertiary care setting over one year and two months
(2015-16). All serologically confirmed dengue patients
(either NS1Ag positivity, if admitted within first 5 days of
fever, or IgM positivity, if after 5 days of fever) between
1 mo-12 y without co-morbidities or co-infections were
enrolled by consecutive sampling. At admission, baseline
history, clinical examination and laboratory investigations
(total count, hematocrit, platelet counts, liver and renal
function tests) were recorded. Close monitoring was done to
note the time of onset of warning signs and severe dengue if
any and need for administration of intravenous fluids till
discharge or death. Daily examination for clinical fluid
accumulation, hepatomegaly, hematocrit and platelet count
were done in all patients. In case of rising hematocrit,
intravenous fluids were started, titrated (as per WHO 2012
guidelines) and hematocrit repeated. In patients with
clinical worsening, 4 hourly hematocrit, 12 hourly platelet
count, and 2 hourly clinical examinations were done, as per
hospital protocol. Ethical clearance was obtained from
Institutional Review Board.
Primary outcome was time duration from
onset of first warning signs to onset of severe dengue
defined as attainment of either severe plasma leak leading
to shock and/or fluid accumulation with respiratory
distress, severe bleed or severe organ impairment [3]. Seven
WHO, 2009 warning signs (dichotomized as yes/no) were:
abdo-minal pain (severe enough to warrant medical
attention), lethargy (without altered sensorium), persistent
vomiting ( ³2
episodes of vomiting that amounts to fatigue or requires
intravenous fluids), mucosal bleed (any bleed from
gastrointestinal/genitourinary mucosa, nose, conjunctiva),
clinical fluid accumulation (either pleural effusion not
severe enough to cause respiratory distress as evidenced by
reduced intensity of breath sounds on auscultation of
axillary areas or ascites as evidenced by shifting
dullness), hepatomegaly >2 cm, hematocrit
³0.40
(cut-off decided by constructing a receiver operating
characteristic curve) and a fall in platelet count <100×109/L
[6].
Sample size for number of events in each
group in survival analysis was calculated where in
d is
natural logarithm of the expected ratio of hazards at a
given time [8]. For a two-tailed test (a
0.05 and b
0.2), by keeping
d
arbitrarily as 1.6, number of events (severe dengue) needed
in each group was calculated as 71; by keeping
d
arbitrarily as 2, events needed in each group was 33.
Statistical analyses: Descriptive
statistics and time to event data analysis were performed
with SPSS version 20. Univariate analysis was done for each
warning signs with time taken for progression to severe
dengue as outcome; Kaplan Meier graphs were drawn. Predictor
significance for inclusion in the multivariate model was
predetermined ( a
20%). Cox proportional hazards model was
checked by looking for parallel lines with and without each
predictor in scatter plots with log time along X-axis
and -log [-log (Survival function)] along Y-axis [9].
RESULTS
Among 386 serologically confirmed dengue
patients, 9 had co-morbidities, 8 had co-infections, 7 did
not have any warning signs and 2 had onset of severe dengue
before onset of the first warning signs. They were excluded
and among remaining 350, 90 (25.7%) progressed to severe
dengue (event); 4 patients with severe dengue died. Remained
260 children (74.3%) did not progress to severe dengue and
were considered ‘right censored’ in time to event analysis.
Median (IQR) age of study population was
7.75 (4.75, 10.25) year. There were 21 infants and 188
(53.7%) were males. Proportion of children who progressed to
severe dengue as evidenced by compensated shock, decompen-sated
shock, respiratory distress, severe bleed and severe organ
impairment as per WHO definitions were 23.1%, 16%, 4.6%,
1.4% and 4.6%, respectively. Median (IQR) day of admission
to our center was on day 5 (4, 6). 154 subjects were NS1Ag
positive, 163 were IgM positive and 33 were both positive;
22.1%, 29.4% and 24.2% progressed to SD respectively. Median
(IQR) length of follow-up was 5 (4, 6) days (Table
I).
Table I Time of Onset of Warning Sign and Time of Onset of Severe Dengue (N=350)
|
Characteristic |
Abdominal |
Persistent |
Lethargy |
Hepatomegaly |
Clinical fluid |
Mucosal |
Platelet count
|
Hematocrit
|
|
pain |
vomiting |
|
>2cm |
accumulation |
bleed |
<100×109/L |
≥ 0.40 |
|
Total with WS* |
217
|
99
|
327
|
162
|
64
|
72
|
284
|
123
|
|
(62) |
(28.2) |
(93.4) |
(46.2) |
(18.2) |
(20.5) |
(81.1) |
(35.1) |
|
Time of onset |
72 |
24 |
6 |
144 |
144 |
132 |
120 |
144
|
|
of WS (h) |
(6,120) |
(6,120) |
(6,72) |
(120,168) |
(144,168) |
(96,162) |
(120,144) |
(120,168) |
|
Total with WS |
211 |
98
|
326
|
143
|
46
|
56
|
270
|
113
|
|
before event*
|
(60.2) |
(28) |
(93.1) |
(40.8) |
(13.1) |
(16) |
(77.1) |
(32.2 ) |
|
Total events*
|
58
|
35 |
86 |
36 |
26 |
21 |
69
|
42
|
|
(16.5) |
(10) |
(24.5)
|
(10.2) |
(7.4)
|
(6) |
(19.7) |
(12) |
|
Time to onset of
|
48
|
120
|
120
|
2
|
2
|
24
|
18
|
5
|
|
event after WS (h) |
(6,120) |
(24,144) |
(48,144) |
(2,3) |
(1,4) |
(4,48) |
(2,24) |
(2,24) |
|
Values in median (IQR) except *n(%); WS-warning
sign. |
Table II Children With Each Warning Sign Who Progressed to Severe Dengue (Event) and Event Free Time
|
Warning sign |
Total |
Events |
Survival Time |
P value |
Crude OR
|
|
|
n= 90 |
(95% CI), min
|
|
(95% CI) |
|
Yes |
211 |
58 (153) |
359.7 (328.98-390.43) |
0.87 |
1.04 |
|
No |
139 |
32 (107) |
324.1 (291.19-357.02) |
|
(0.67-1.59) |
|
Lethargy
|
|
|
|
|
|
|
Yes |
326 |
86 (240) |
362.5 (337.46-387.60) |
0.69 |
1.26 |
|
No |
24 |
4 (20) |
167.2 (141.90-192.59) |
|
(0.39-3.99) |
|
Persistent vomiting |
|
|
|
|
|
|
Yes |
98 |
35 (63) |
276.4 (242.73-310.04) |
0.13 |
1.38
|
|
No |
252 |
55 (197) |
384.1 (356.53-411.64) |
|
(0.90-2.10) |
|
Clinical fluid accumulation |
|
|
|
|
|
|
Yes
|
46 |
26 (20) |
178.3 (146.04-210.48) |
0.002 |
2.19 |
|
No |
304 |
64 (240) |
379.1 (353.64-404.59) |
|
(1.33-3.59) |
|
Hepatomegaly
|
|
|
|
|
|
|
Yes |
143 |
36 (107) |
363.8 (339.18-388.35) |
0.81 |
1.01 |
|
No
|
207 |
54 (153) |
370.7 (338.81-402.67) |
|
(0.69-1.59) |
|
Mucosal bleed |
|
|
|
|
|
|
Yes |
56 |
21 (35) |
204.8 (177.67-231.89) |
0.14 |
1.45 |
|
No |
294 |
69 (225) |
370.3 (342.62-398.01) |
|
(0.89-2.36) |
|
Hematocrit ³0.40 |
|
|
|
|
|
|
Yes |
113 |
42 (71) |
265.5 (232.60-298.35) |
0.009 |
1.71 |
|
No
|
237 |
48 (189) |
392.3 (364.75-419.91) |
|
(1.13-2.59) |
|
Platlet count <100x109/L |
|
|
|
|
|
|
Yes |
270 |
69 (201) |
314.6 (291.83-337.30) |
0.97 |
1.01
|
|
No |
80 |
21 (59) |
382.0 (333.96-430.14) |
|
(0.61-1.66) |
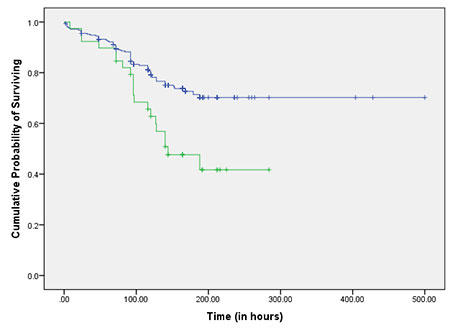 |
|
Fig. 1 Kaplan Meier Curve showing survival
function over time in the absence (upper line) and
presence (lower line) of CFA as predictor.
|
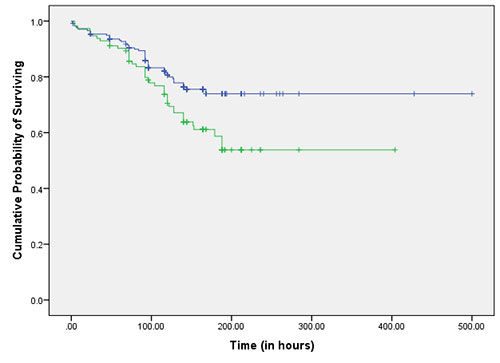 |
|
Fig. 2 Kaplan Meier curve
showing survival function over time in the absence
(upper line) and presence (lower line) of HCT >0.40
as predictor.
|
Log rank test was applied to the data and
Kaplan Meier curves were drawn to compare between groups
with and without each warning sign (Table
II, Fig. 2a, 2b).
Final model includes all warning signs with P<0.2 in
univariate analysis (clinical fluid accumulation, mucosal
bleed, persistent vomiting and hematocrit
³0.40) (Table
III).
Table III Cox Proportional Hazards Model With Selected Warning Signs
|
Warning
|
Model including CFA,HCT
≥0.40, PV |
Model including CFA, HCT≥0.40 |
|
signs |
|
and MB |
|
and MB
|
|
|
HR (95% CI) |
P value |
HR (95% CI) |
P value
|
|
CFA |
1.89 (1.11-3.20) |
0.02 |
1.85 (1.09-3.12) |
0.02 |
|
Hct ³0.40 |
1.49 (0.96-2.29) |
0.07 |
1.54 (1.00-2.35) |
0.05 |
|
MB |
1.17 (0.69-1.97) |
0.56 |
1.25 (0.76-2.07) |
0.38 |
|
PV |
1.25 (0.80-1.95) |
0.32 |
- |
- |
|
CFA: Clinical fluid accumulation; Hct:
Hematocrit; PV: Persistent vomiting; MB: Mucosal
bleed; HR: Hazard ratio. |
Receipt of intravenous fluids could
confound time taken for progression to severe dengue, but
statistical significance was not obtained in univariate
analysis with time to event as outcome.
DISCUSSION
The study shows that clinical fluid
accumulation, hematocrit
³0.40,
mucosal bleed and persistent vomiting predict time taken for
progression to severe dengue. Earlier, authors developed a
prognostic prediction model to determine severe dengue in
children that included clinical fluid accumulation
hematocrit ³0.40
with platelet count <100×109/L
and persistent vomiting [6].
In the present study, clinical fluid
accumulation appeared late with a median time of onset of
144 h from onset of fever. Moreover, median time of onset of
severe dengue is only 2h from onset of clinical fluid
accumulation. In most situations, authors were the first to
identify clinical fluid accumulation; being a tertiary
setting, exact time of onset of clinical fluid accumulation
could not be delineated. In our study, hematocrit appeared
late probably because the investigation was not sent before
admission to our center. Even then, median time of onset of
severe dengue was 5h after onset of hematocrit
³0.40.
This time gap is clinically valuable for initiating close
monitoring, intensive care and early referral if needed.
This makes hematocrit ³0.40
a clinically relevant warning signs. Kaplan Meier curves
drawn for clinical fluid accumulation and hematocrit
³0.40 as
predictors intersect at some points. Hence confounders do
exist for which stratum specific analysis might have been
helpful. Administration of intravenous fluids was thought of
as a potential confounder but statistical significance was
not obtained in univariate analysis. Possibility of unknown
confounders should be thought of in this context.
Mucosal bleed and persistent vomiting are
two objective symptoms, time of onset of which the caretaker
may easily notice. An added advantage of persistent vomiting
is its early appearance in the disease course. A sufficient
time gap between time of onset of persistent vomiting and
time of onset of severe dengue was also demonstrated in our
study. Due to these clinical reasons, mucosal bleed and
persistent vomiting were included in the final model.
In our tertiary care setting, some
patients had onset of warning signs even before admission to
our hospital. To minimize this recall bias, details from
referral letters were collected and telephonic conversations
with referring doctor were done wherever needed. Though
technically, 260 patients were right censored, all were
completely followed up till recovery as evidenced by fever
free period of 48 hours, disappearance of clinical warning
signs, rising trend of platelet counts and a normal
hematocrit. Secondary infection is a strong risk factor of
progression to severe dengue and hence may influence time to
event. Detailed investigations to delineate infection as
primary or secon-dary were not done in our study. Our study
period included two dengue seasons, but only 90 patients
progressed to severe dengue which was below the estimated
sample size.
A previous survival analysis assessed
survival of adult dengue patients in relation to the
severity of liver dysfunction [10]. Survival analysis of a
pediatric popu-lation has identified that acute renal
failure adversely affects survival rates [11]. In these
studies, event was mortality whereas in our study, event
severe dengue. Lam, et al. [7] have found that
prediction models with serial daily platelet counts
demonstrated better ability to discriminate patients who
developed shock than models based on enrolment information
only [7]. They concluded that development of dynamic
prediction models that incorporate signs, symptoms and daily
laboratory measure- ments could improve dengue shock
prediction. In our study, all seven WHO warning signs have
been included for the purpose of prediction.
Our results may be generalized to
children attending a health care facility with dengue. As
India is hyper-endemic for dengue, the study results have
implications in policy making and practice guidelines,
especially to triage children attending a health care
facility with or without warning signs. To conclude, WHO
warning signs that can predict time taken for progression to
severe dengue in children include clinical fluid
accumulation, hematocrit
³0.40,
persistent vomiting and mucosal bleed.
Acknowledgements: Dr Sasikala K,
Director, CERTC, Govern-ment Medical College,
Thiruvananthapuram for the conduct of this study.
Ethics clearance: Institutional
Review Board, Government Medical College, Thiruvananthapuram;
No. 06/62/2015/MCT, dated December 09, 2015.
Contribution: PS: conceived the idea,
designed the metho-dology, collected and analysed data and
prepared the manuscript; GS: guided conduct of the study,
critically reviewed the manuscript; SKA: elaborated the
concept, interpreted the results, critically reviewed the
manuscript and approved final version to be published. All
authors approved the final version of manu-script, and are
accountable for all aspects related to the study.
Funding: None; Competing interests:
None stated.
|
What This Study Adds?
• WHO warning signs that predict time taken for
progression to severe dengue in children include
clinical fluid accumulation, hematocrit
³0.40,
persistent vomiting and mucosal bleed.
|
REFERENCES
1. World Health Organization. Dengue and
Severe Dengue. Available from https://www.who.int/news-room/fact-
sheets/detail/dengue-and-severe-dengue/. Accessed July
25, 2019.
2. National Vector Borne Disease Control
Programme. Dengue. Dengue cases and deaths in the country
since 2010. Available from
https://www.nvbdcp.gov.in/den-cd.html/. Accessed July
25, 2019.
3. World Health Organization. Dengue
Guidelines for diagnosis, treatment, prevention and control:
New edition 2009. Available from:
https://www.who.int/rpc/guidelines/9789241547871/en/.
Accessed July 25, 2019.
4. Alexander N, Balmaseda A, Coelho ICB,
Dimaano E, Hien TT, Hung NT, et al. Multicentre
prospective study on dengue classification in four
South-east Asian and three Latin American countries. Trop
Med Inter Health. 2011; 16:936-48.
5. DinhThe T, Le Thi Thu T, Nguyen Minh
D, Tran Van N, Tran Tinh H, Nguyen Van Vinh C, et al.
Clinical features of Dengue in a large Vietnamese cohort:
Intrinsically lower platelet counts and greater risk for
bleeding in adults than children. PLoSNegl Trop Dis.
2012;6:e1679.
6. Sreenivasan P, S Geetha, K Sasikala.
Development of a prognostic prediction model to determine
severe dengue in children. Indian J Pediatr. 2018;85:433-39.
7. Lam PK, Ngoc TV, Thu Thuy TT, Hong Van
NT, NhuThuy TT, Hoai Tam DT, et al. The value of
daily platelet counts for predicting dengue shock syndrome:
Results from a prospective observational study of 2301
Vietnamese children with dengue. PLoS Negl Trop Dis.
2017;11: e0005498.
8. Norman GR and Streiner DL.
Nonparametric statistics. Life Table (Survival Analysis).
In: Norman GR and Streiner DL, editors. Biostatistics. The
bare essentials. Ontario: B.C Decker Inc; 1998. P.182-94.
9. Bradburn MJ, Clark TG, Love SB and
Altman DG. Survival analysis Part III: Multivariate data
analysis- choosing a model and assessing its adequacy and
fit. Br J of Cancer. 2003;89:605-11.
10. Hanif A, Butt A, Ahmed A, Sajid MR,
Ashraf T, Nawaz AA. Survival analysis of Dengue patients in
relation to severity of liver dysfunction in Pakistan. Adv
Biolog Res. 2015;9:91-94.
11. Basu B, Roy B. Acute renal failure adversely affects
survival in pediatric Dengue infection. Indian J Crit Care
Med. 2018;22:30-33.
|
|
|
 |
|

