|
|
|
Indian Pediatr 2012;49:
793-798 |
 |
Immunogenicity and Safety of a DTaP-IPV//PRP~T
Vaccine (Pentaxim) Booster Dose During the Second Year of Life
in Indian Children Primed with the Same Vaccine
|
|
AK Dutta, *VP Verghese, H Pemde , *LG Mathew and
‡E Ortiz
From the Lady Hardinge Medical College and Associated
Hospitals, New Delhi, India; *Christian Medical College Hospital,
Vellore, Tamil Nadu; India, and ‡Sanofi Pasteur, Lyon, France.
Correspondence to: Dr Esteban Ortiz MD, Global Medical
Affairs, Sanofi Pasteur, 2 avenue Pont Pasteur,
69007, Lyon, France.
Email:
[email protected]
Received: November 04, 2011;
Initial review: December 07, 2011;
Accepted: January 12, 2012.
Published online: 2012, March 30.
PII:S097475591100916 - 1
|
Objective: To evaluate the immunogenicity and safety of a
pentavalent (diphtheria, tetanus, acellular pertussis, inactivated
poliovirus, Hib polysaccharide-conjugate) combination vaccine booster
dose.
Design: Multicenter, open, Phase III clinical
study.
Setting: Two tertiary-care hospitals in
Delhi and Vellore, India.
Participants/patients: 207 healthy Indian
children.
Intervention: The DTaP-IPV//PR~NT vaccine (Pentaxim)
was given at 18-19 months of age to children who had been primed with
the same vaccine at 6,10,14 weeks of age.
Main outcome measures: Immunogenicity was
assessed before and 1 month after the booster. Safety was evaluated from
parental reports, and investigator assessments.
Results: At 18-19 months of age, before boosting,
the SP rates against diphtheria, tetanus, poliovirus and PRP were
82.3-100%; 90.0% of participants had anti-PRP
≥0.15
µg/mL. Anti-poliovirus titers were ≥1:8
dilution in 97.9-98.4% of participants. Anti-PT and FHA titers (≥5
EU/mL) were detectable in 82.5% and 90.8% of participants, respectively.
One month after the booster dose, SP rates were 99.5% for PRP (≥1.0
µg/mL), 100% for diphtheria, tetanus (≥0.1
IU/mL) and polioviruses (≥8:1/dilution).
Sero-conversion (4 fold post-booster increase in anti-PT and -FHA
concentration) occurred in 96.8% and 91.7%, respectively. Geometric mean
concentrations (GMC) increased from 11.7 to 353.1 EU/mL and from 18.2 to
363.4 EU/mL for anti-PT and anti-FHA, respectively. Anti-PRP GMC
increased from 1.75 to 70.5 µg/mL. Vaccine reactogenicity was low;
severe solicited reactions were reported by <1.4% of participants.
Conclusion: The DTaP-IPV//PRP-T vaccine booster
at 18-19 months of age was well tolerated and induced strong antibody
responses.
Key words: Antibody persistence, Booster
vaccination, Efficacy, Immunogenicity, Pentavalent vaccine, Safety.
|
|
T
he Indian Academy of Pediatrics (IAP) recommends
Haemophilus influenzae type b (Hib) vaccination and IPV for all
children [1]. Booster doses of many childhood vaccines, including
pertussis, Hib and polio are included in many national programmes during
the second year of life [2]. The primary reasons for this are
persistence of pertussis and Hib disease in children in countries
without routine booster vaccinations, and observation that
vaccine-induced immunity wanes over time, especially when an infant
primary series is not followed-up with a toddler booster vaccination
[2-4]. The WHO recommends a pertussis booster for children aged 1-6
years, preferably during the second year of life, with the primary
series plus booster expected to ensure protection for 6 years [5].
The safety and immunogenicity of DTaP-IPV/1PRP~T
vaccine (Pentaxim) have been assessed previously [6,7]. This study
evaluated the immunogenicity, and safety of a DTaP-IPV//PRP~T booster
vaccination administered at 18-19 months of age in a group of children
who had been given a three dose primary series vaccination of the same
vaccine at 6, 10, and 14 weeks of age and monovalent hepatitis B (HB)
vaccine at birth, 6 and 14 or 6, 10 and 14 weeks of age [8].
Methods
This Phase III, open clinical study was performed at
Lady Hardinge Medical College and Associated Hospitals in New Delhi and
Christian Medical College Hospital, Vellore, Tamil Nadu. The study
protocol and consent form were approved by each institutional review
board. The study conformed to local regulations, Good Clinical Practices
(GCP) and applicable International Conference on Harmonization (ICH)
guidelines and the ethical principles of the Declaration of Helsinki.
Written informed consent was obtained from a parent/legal guardian of
each participant before enrolment.
Healthy full-term ( ³37
weeks) infants weighing ³2.5 kg
at birth who had completed primary vaccination with the DTaP-IPV//PRP~T
vaccine at 6, 10, and 14 weeks of age [8] were eligible for booster
vaccination with the same vaccine at 18-19 months of age. The booster
phase was conducted from July 2007 to April 2008. The objectives were to
measure antibody persistence prior to the booster dose and the immune
response 1 month post-booster.
The composition of each 0.5 mL dose of the DTaP-IPV//PRP~T
study vaccine (Pentaxim, Sanofi Pasteur, France, batch number A2053) is
described elsewhere [6, 8]. The lyophilized PRP~T antigen was
reconstituted with the liquid DTaP-IPV vaccine immediately before IM
injection into the anterolateral aspect of the upper right thigh. Blood
samples (4 mL) were collected for antibody determination just before,
and 4-6 weeks after the booster. Serologic analyses were performed at
Sanofi Pasteur’s Global Clinical Immunology central laboratory in
Swiftwater, Pennsylvania, USA, using analysis methods described
elsewhere [8]. The predefined antibody levels for seroprotection (SP)
were: anti-PRP ≥0.15
and ≥1.0 µg/mL,
anti-poliovirus ≥ 8
(1/dilution), anti-diphtheria ≥0.01
and ≥0.10 IU/mL,
anti-tetanus ≥0.01
and ≥0.10 IU/mL.
Seroconversion (SC) for anti-pertussis antigens was defined as a
≥4-fold increase in
antibody concentration post-vaccination [9].
Investigators monitored each participant for
immediate adverse events for 30 minutes after vaccination. Parents/legal
guardians recorded, and graded the severity of, solicited injection site
(redness, swelling and tenderness) and systemic (fever - axillary
temperature ³37.4ºC,
vomiting, abnormal crying, drowsiness, loss of appetite and
irritability) reactions on diary cards for 8 days after vaccination.
Unsolicited reactions were recorded, with onset date, intensity and
resolution, for 30 days after vaccination. Serious adverse events (SAEs)
were reported throughout the study.
Statistical analysis: SP and SC rates were
calculated with 95% confidence intervals (CIs) using the exact binomial
method. Geometric mean titers (GMTs) and concentrations (GMCs) were
calculated with 95% CIs using the normal approximation. Reverse
Cumulative Distribution Curves (RCDCs) for pre- and post-vaccination
antibody titers were derived for each antibody response.
Results
Of the 216 participants who completed the primary
series, three withdrew voluntarily before the booster was given, one was
lost to follow up, and five had protocol violations (received a
non-study DTP vaccine). The remaining 207 participants received the
booster injection and provided the first blood sample. One additional
participant withdrew voluntarily before collection of the second blood
sample and was excluded from the post-booster immunogenicity analysis
set presented here. All 207 participants given the booster vaccination
were included in the safety analysis set.
Immunogenicity: Seroprotection rates were
high at 18-19 months of age when the booster dose was given (Table
I). At least 97.9% of the participants still had anti-tetanus
concentrations ≥0.01
IU/mL and poliovirus titers ≥8
(1/dilution). Anti-diphtheria concentrations ≥0.01 IU/mL and
anti-PRP concentrations ≥0.15
µg/mL were still observed in 82.3% and 90.0% of participants,
respectively. Following booster vaccination, SP rates against diphtheria
and tetanus (≥0.1
IU/mL) and poliovirus (≥8
1/dilution) were 98.0 to 100%; anti-PRP titers ≥1.0 µg/mL were
observed in 99.5% of participants, and at least 91.7% of participants
seroconverted against PT and FHA.
TABLE I Seroprotection and Seroconversion Rates for Each Antigen at 1 month Post-primary,
Pre-booster and 1 month Post-booster Vaccination
|
Criteria |
Post-primary |
Pre-booster |
Post-booster |
|
%
(95% CI) |
%
(95% CI) |
%
(95% CI) |
| Anti-PRP
≥0.15 µg/mL |
98.5 (95.7;
99.7) |
90.0 (85.0;
93.8) |
100.0 (98.2;
100.0) |
| Anti-PRP
≥1.0 µg/mL |
89.6 (84.5;
93.4) |
60.0 (52.9;
66.8) |
99.5 (97.3;
100.0) |
|
Anti-Diphtheria ≥0.01 IU/mL |
99.0 (96.5;
99.9) |
82.3 (76.3;
87.4) |
100.0 (98.2;
100.0) |
|
Anti-Diphtheria ≥0.10 IU/mL |
18.3 (13,2;
24.4) |
14.1 (9.6;
19.8) |
98.0 (95.0;
995) |
| Anti-Tetanus
≥0.01 IU/mL |
100.0 (98.2;
100.0) |
100.0 (98.0;
100.0) |
100.0 (98.2;
100.0) |
| Anti-Tetanus
≥0. 10 IU/mL |
100 (98.2;
100.0) |
84.2 (78.2;
89.2) |
100 (98.2;
100.0) |
| Anti-Polio 1
≥8 1/dil. |
100.0 (98.2;
100.0) |
98.4 (95.5;
99.7) |
100.0 (98.2;
100.0) |
| Anti-Polio 2
≥8 1/dil. |
99.0 (96.5;
99.9) |
97.9 (94.6;
99.4) |
100.0 (98.1;
100.0) |
| Anti-Polio 3
≥8 1/dil. |
100.0 (98.2;
100.0) |
98.4 (95.5;
99.7) |
100.0 (98.1;
100.0) |
| Anti-PT
≥4-fold increase |
94.4 (90.2;
97.2)* |
|
96.8 (93.2;
98.8)† |
| Anti-FHA
≥4-fold increase |
86.0 (80.4;
90.5)* |
|
91.7 (86.8;
95.2)† |
|
*Increase from pre-to post-priming; †Increase from pre-booster. |
TABLE II Geometric Mean Concentrations (GMCs) and Titers (GMTs) for each Antigen at
1 month Post-primary, Pre-booster and 1-month Post-booster Vaccination
|
Post-primary |
Pre-booster |
Post-booster |
Post-/pre-booster |
|
GMC*
or GMT† |
GMC
or GMT |
GMC
or GMT |
GMR(95% CI) |
|
(95% CI) |
(95% CI) |
(95% CI) |
|
| Anti-PRP µg/mL |
4.19
(3.52;4.98) |
1.75
(1.34;2.29) |
70.56
(60.22;82.67) |
39.7
(29.85;52.7) |
|
Anti-Diphtheria IU/mL |
0.046
(0.040;0.053) |
0.028
(0.023;0.034) |
3.940
(3.286;4.723) |
141.3
(117.2;170.4) |
| Anti-Tetanus
IU/mL |
0.93
(0.86;1.00) |
0.29
(0.24;0.34) |
13.91(12.51;15.46) |
48.0
(39.8;57.8) |
| Anti-Polio 1
(1/dil) |
435.7
(359.4;528.3) |
334.4
(249.6;448.1) |
7777.0
(6705.8;9019.3) |
25.4
(18.8;34.2) |
| Anti-Polio 2
(1/dil) |
447.9
(349.9;573.2) |
357.4
(263.0;485.7) |
8638.3
(7352.4;10149.1) |
26.8
(19.0;37.7) |
| Anti-Polio 3
(1/dil) |
1488.3
(1255.6;1764.0) |
271.9
(207.1;357.0) |
11523.6
(9785.4;13570.7) |
50.4
(37.8;67.2) |
| Anti-PT EU/mL |
324.2
(296.0;355.1) |
11.7
(10.1;13.6) |
353.1
(320.9;388.6) |
29.7
(25.4;34.7) |
|
Anti-FHA EU/mL |
92.8 (83.8;102.8) |
18.2 (15.1;21.9) |
363.4 (324.2;407.3) |
20.3 (17.0;24.4) |
|
*Geometric mean concentration: Anti-PRP, Anti-Tetanus,
Anti-Diphtheria, Anti-PT, Anti-FHA; †Geomentric mean titer,
Anti-Polio; GMR=geometric mean ratio. |
GMTs decreased between the primary series and booster
administration (Table II); however, at least 90.0% of
participants were still seroprotected against tetanus (≥0.01
IU/mL), the three polioviruses (≥8
1/dilution), and Hib (anti-PRP ≥0.15
µg/mL). Seroprotective anti-diphtheria antibody concentrations (≥0.01
IU/mL) were observed in the majority of participants, although the
percentage was lower than for other antigens. Anti–PT and anti- FHA
concentrations ≥5
EU/mL were observed in 82.5% and 90.8% of participants, respectively
(data not shown). Figure 1 shows strong, linear increases
for anti-PT,-FHA, -PRP, and all three -polioviruses.
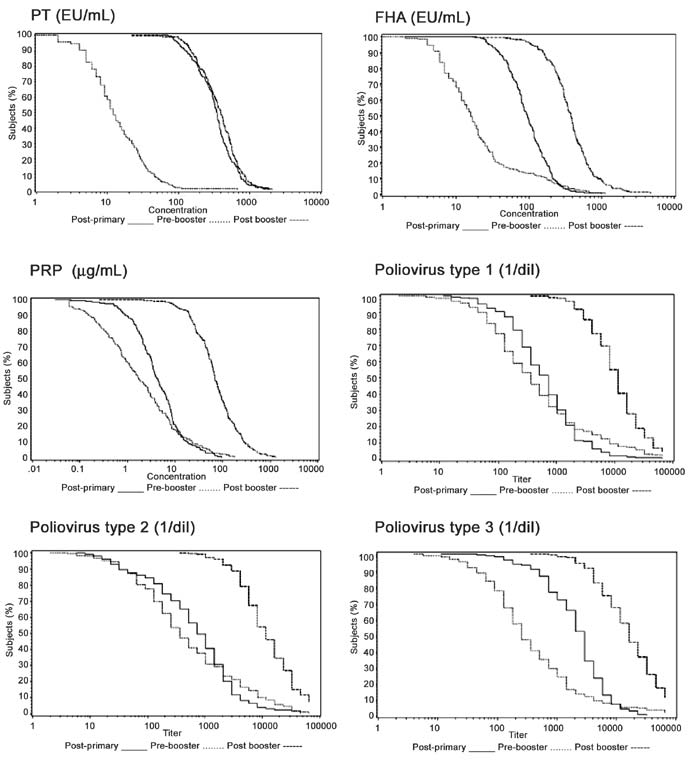 |
|
Fig.1 Reverse cumulative distribution
curves for PT, FHA, PRP, and poliovirus 1, 2, and 3 after a
3-dose primary series, and before and after a booster
vaccination.
|
Reactogenicity and safety: 87 of the 207
participants (42.0%) reported a solicited reaction within 8 days of
vaccination. Most occurred within three days and resolved without
treatment. The most frequent injection site reaction was tenderness
(21.7%) and the most frequent systemic reactions was fever (19.3%) (Table
III). Unsolicited events were reported by 27 participants (13%).
Most were infections (11.1% of participants) with upper respiratory
tract infections (8.2% of participants) predominating. A single SAE was
reported - a case of lobar pneumonia that resolved after treatment.
TABLE III
Solicited Reactions in Available Infants (N=207) Within 8 Days After a Booster Dose Given at 18-19 Months of Age
|
|
|
Number |
%
(95%CI) |
|
Injection site reactions |
|
Tenderness |
Any |
45 |
21.7 (16.3;
28.0) |
|
|
Severe |
2 |
1.0 (0.1;
3.4) |
|
Redness |
Any |
18 |
8.7 (5.2;
13.4) |
|
|
Severe |
1 |
0.5 (0.0;
2.7) |
|
Swelling |
Any |
23 |
11.1 (7.2;
16.2) |
|
|
Severe |
0 |
0.0 (0.0;
1.8) |
|
Systemic reactions |
|
Fever |
Any |
40 |
19.3 (14.2;
25.4) |
|
|
Severe |
3 |
1.4 (0.3;
4.2) |
|
Vomiting |
Any |
15 |
7.2 (4.1;
11.7) |
|
|
Severe |
1 |
0.5 (0.0;
2.7) |
|
Abnormal
crying |
Any |
22 |
10.6 (6.8;
15.6) |
|
|
Severe |
1 |
0.5 (0.0;
2.7) |
|
Drowsiness |
Any |
18 |
8.7 (5.2;
13.4) |
|
|
Severe |
0 |
(0.0; 2.7) |
|
Loss of
appetite |
Any |
20 |
9.7 (6.0;
14.5) |
|
|
Severe |
1 |
0.5 (0.0;
2.7) |
|
Irritability |
Any |
25 |
12.1 (8.0;
17.3) |
|
|
Severe |
0 |
0.0 (0.0;
1.8) |
|
% = percentage of participants with a
specific adverse event. Mild, moderate or severe tenderness:
‘minor reaction when injection site is touched’, ‘cries and
protests when injection site is touched’, and ‘cries when
injected limb is moved, or the movement of the limb is reduced’.
Erythema and swelling: a diameter of <2.5 cm was mild, 2.5-5 cm
was moderate and >5 cm was severe. Mild, moderate and severe
fever: axillary temperatures
³37.4ºC to
37.9ºC, ³38ºC
to 38.9ºC, and ³39ºC,
respectively.
|
Discussion
This study evaluated the immunogenicity and safety of
a DTaP-IPV//PRP~T vaccine booster at 18-19 months of age in participants
who had completed a primary series vaccination at 6, 10, 14 weeks of age
with the same vaccine given with a monovalent HB vaccine. The results
following booster vaccination in this study population are consistent
with previous studies of this pentavalent vaccine using various
schedules, including the EPI schedule followed here [6].
The very high SP rates observed here for each vaccine
antigen after the booster dose, and the large increases in GMCs/GMTs,
are consistent with long-term protection. The waning of anti-PT and
anti-FHA serum antibody concentrations followed by a strong booster
response as seen here is well documented [10,11]. Similar results have
been previously reported with this and other DTaP-combined vaccines
[6,12]. In this study, the post-booster SC rates of 96.8% and 91.7% for
anti-PT and anti-FHA as well as the large increases in other antibody
GMCs and GMTs are indicative of strong anamnestic immune responses. The
anti-poliovirus antibody persistence and strong IPV booster response
observed here provide additional immunogenicity data to support IPV
administration in a 6, 10, 14 week EPI schedule with a booster at 18-19
months of age.
High vaccine effectiveness of DTaP combination
vaccines containing conjugated Hib antigens has been demonstrated in
Europe [13,14,15]. In Sweden, where the study vaccine has been in the
National Program since 1997, the incidence of invasive Hib disease was
0.5/100,000 in 1997 and 0.16/100,000 in 2008 [13]. Pertussis
surveillance in Sweden revealed that vaccination at 3, 5 and 12 months
of age since 1997 resulted in a marked decrease in pertussis incidence
compared to no vaccination. Protection has remained high for 5-7 years
after the third (booster) dose, when an additional booster dose is now
recommended [16,17,18]. Although the schedule followed in India is
different, we believe that the Swedish surveillance data are applicable
because of the high immunogenicity of this vaccine across a range of
primary series and booster vaccination schedules [6,7].
Acellular pertussis vaccines are generally better
tolerated than DTwP combinations for both primary and booster
vaccination, but the occurrence and severity of injection site reactions
tend to increase with each successive dose of either vaccine [2,5,6].
Although the incidence of solicited adverse reactions in this study was
slightly higher than seen with primary vaccination, the overall
reactogenicity of the booster dose indicates it was well tolerated.
Severe injection site reactions occurred in no more than 7.2% of
participants; no severe solicited systemic event was reported by more
than 3.3%. No hypotonic-hyporesponsive episode or seizure was reported,
and no participant withdrew because of a vaccination-related AE.
This study confirms that the booster at 18-19 months
of age with the study vaccine was appropriately timed (with pre-booster
antibody titers being satisfactory), well tolerated, and induced strong
antibody responses to all the vaccine antigens.
Acknowledgments: Fabrice Guitton and
Ranjeet Kaur for study monitoring, Roy Fernando for data management and
Valérie Bosch-Castells for statistical analysis. The authors would also
like to thank Clement Weinberger (Le Stylo Communications) and Andrew
Lane for assistance with the draft manuscript preparation. FG, RK, RF,
VB-C, and AL are employees of Sanofi Pasteur.
Contributors: AKD, VPV, HP and LGM:
responsible for study conduct, data acquisition, data interpretation,
manuscript review and approval; EO: responsible for study design, data
interpretation, manuscript review and approval.
Funding: This study was conducted with the
financial support of Sanofi Pasteur, Lyon, France
Competing interests: EO is employee of Sanofi
Pasteur, which manufactures the vaccine evaluated in this paper.
|
What is Already Known?
•
DTaP-IPV//PRP~T vaccine shows
good antibody persistence in the second year of life and is safe
and immunogenic when administered as a booster during the second
year of life.
What This Study Adds?
• Additional persistence and booster
immunogenicity and safety data for a DTaP-IPV//PRP~T vaccine
following vaccination at 18-19 months of age in Indian children,
who had received a primary series with the same vaccine at 6,10,
and 14 weeks.
|
References
1. IAP Committee on Immunization Consensus
Recommendations on Immunization, 2008. Indian Pediatr. 2008;45:635-48.
2. Edwards K, Decker M. Pertussis vaccines. In:
Plotkin S, Orenstein W, Offit PA, eds. Vaccines. 5th Edition.
Philadelphia PA. Saunders: 2008; p. 467-517.
3. Barret AS, Ryan A, Breslin A, Cullen L, Murray A,
Grogan J, et al. Pertussis outbreak in northwest Ireland, January
- June 2010. Euro Surveill. 2010;15. pii: 19654.
4. Johnson NG, Ruggeberg JU, Balfour GF, Lee YC,
Liddy H, Irving D, et al. Haemophilus influenzae type b
reemergence after combination immunization. Emerg Infect Dis.
2006;12:937-41.
5. World Health Organization. Pertussis vaccines WHO
position paper. Weekly Epidemiol Rec. 2010; 85:385-400.
6. Plotkin SA, Liese J, Madhi SA, Ortiz E. A DTaP-IPV//PRP~T
vaccine (Pentaxim™): a review of 16 years’ clinical experience. Expert
Rev Vaccines. 2011;10:981-1005.
7. Vidor E, Plotkin SA. Immunogenicity of a
two-component (PT & FHA) acellular pertussis vaccine in various
combinations. Hum Vaccine. 2008;4:328-40.
8. Dutta AK, Verghese VP, Pemde HK, Mathew LG, Ortiz
E. Immunogenicity and safety of a pentavalent diphtheria, tetanus,
acellular pertussis, inactivated poliovirus, Haemophilus influenzae
type B conjugate combination vaccine (Pentaxim) with hepatitis B
vaccine. Indian Pediatr. 2009;46:975-82.
9. Plotkin SA. Correlates of protection induced by
vaccination. Clin Vaccine Immunol. 2010;17:1055-65.
10. Grimprel E, Begue P, Anjak I, Njamkepo E,
Francois P, Guiso N. Long-term human serum antibody responses after
immunization with whole-cell pertussis vaccine in France. Clin Diagn Lab
Immunol. 1996;3:93-7.
11. Guiso N, Njamkepo E, Vie lS, Zepp F, Meyer CU,
Abitbol V, et al. Long-term humoral and cell-mediated immunity
after acellular pertussis vaccination compares favourably with
whole-cell vaccines 6 years after booster vaccination in the second year
of life. Vaccine. 2007;25:1390-7.
12. Tichmann I, Grunert D, Habash S, Preidel H,
Schult R, Pfletschinger U, et al. Persistence of antibodies in
children primed with two different hexavalent diphtheria, tetanus,
acellular pertussis, hepatitis B, inactivated poliovirus and
Haemophilus influenzae type B vaccines and evaluation of booster
vaccination. Hum Vaccin. 2006;2:249-54.
13. Hallander HO, Lepp T, Ljungman M, Netterlid E,
Andersson M. Do we need a booster of Hib vaccine after primary
vaccination? A study on anti-Hib seroprevalence in Sweden 5 and 15 years
after the introduction of universal Hib vaccination related to
notifications of invasive disease. Acta Pathologica Microbiologica et
Immunologica Scandinavica (APMIS). 2010;118:878-87.
14. Kalies H, Verstraeten T, Grote V, Meyer N,
Siedler A, Schmitt HJ, et al. Erhebungseinheit für seltene
pädiatrische Erkrankungen in Deutschland Study Group. Four and
one-half-year follow-up of the effectiveness of diphtheria-tetanus
toxoids - acellular pertussis/Haemophilus influenzae type b and
diphtheria-tetanus toxoids-acellular pertussis-inactivated poliovirus/H.
influenzae type b combination vaccines in Germany. Pediatr Infect
Dis J. 2004;23:944-50.
15. Schmitt HJ, von Kries R, Hassenpflug B, Hermann
M, Siedler A, Niessing W, et al. Haemophilus influenzae
type b disease: impact and effectiveness of diphtheria-tetanus
toxoids-acellular pertussis (-inactivated poliovirus)/H. influenzae
type b combination vaccines. Pediatr Infect Dis J. 2001;20:767-74.
16. Carlsson RM, Trollfors B. Control of pertussis—lessons
learnt from a 10-year surveillance programme in Sweden. Vaccine.
2009;27:5709-18.
17. Gustafsson L, Hessel L, Storsaeter J, Olin P.
Long-term follow-up of Swedish children vaccinated with acellular
pertussis vaccines at 3, 5, and 12 months of age indicates the need for
a booster dose at 5 to 7 years of age. Pediatrics. 2006;118:978-84.
18. Swedish Institute for Infectious Disease Control.
Pertussis surveillance in Sweden with enhanced follow-up of cohorts
immunized with acellular pertussis vaccines 2009 Appendix 2 to
Eleven-year Report.
http://www.smittskyddsinstitutet.se/upload/Publikationer/11-y-report-app202-SPMSD.pdf.
Accessed 3 November, 2011.
|
|
|
 |
|

