|
|
|
Indian Pediatr 2021;58:1077-1084 |
 |
Feasibility of Pediatric
Non-Invasive Respiratory Support in Low- and Middle-Income
Countries
|
|
Krishna Mohan Gulla, Sushil Kumar Kabra, Rakesh Lodha
From Division of Pediatric Pulmonology and Intensive Care, Department
of Pediatrics, All India Institute of Medical Sciences, New Delhi.
Correspondence to: Dr Rakesh Lodha, Professor, Division of Pediatric
Pulmonology and Intensive Care, Department of Pediatrics, All India
Institute of Medical Sciences, New Delhi, Ansari Nagar, New Delhi,110
029.
Email:
[email protected]
Published online: May 03, 2021;
PII: S097475591600320
|
Non-Invasive respiratory support can be viewed as
mechanical respiratory support without endotracheal intubation and it
includes continuous positive airway pressure, bi-level positive airway
pressure, high flow nasal cannula, and non-invasive positive pressure
ventilation. Over past few years, non-invasive respiratory support is
getting more popular across pediatric intensive care units for acute
respiratory failure as well as for long-term ventilation support at
home. It reduces the need for invasive mechanical ventilation, decreases
the risk of nosocomial pneumonia as well as mortality in selected
pediatric and adult population. Unfortunately, majority of available
studies on non-invasive respiratory support have been conducted in
high-income countries, which are different from low- and middle-income
countries (LMICs) in terms of resources, manpower, and the disease
profile. Hence, we need to consider disease profile, severity at
hospital presentation, availability of age-appropriate equipment,
ability of healthcare professionals to manage patients on non-invasive
respiratory support, and cost-benefit ratio. In view of the
relatively high cost of equipment, there is a need to innovate to
develop indigenous kits/ devices with available resources in LMICs to
reduce the cost and potentially benefit health system. In this review,
we highlight the role of non-invasive respiratory support in different
clinical conditions, practical problems encountered in LMICs setting,
and few indigenous techniques to provide non-invasive respiratory
support.
Keywords: Continuous positive airway pressure, High flow nasal
cannula, Low- and middle-income countries, Non-invasive ventilation.
|
|
N on-invasive
respiratory support (NRS) is
defined as delivery of respiratory support
without use of an invasive artificial
airway such as endotracheal or tracheostomy tube. It can be
delivered using negative pressure or positive pressure. In
negative pressure ventilation, pressure surrounding the chest
wall is lowered to decrease intra-pleural pressure and thus,
tidal volume is delivered to patient. Iron lung, which was used
in polio epidemic six decades ago is an example of negative
pressure ventilation [1]. In positive pressure non-invasive
respiratory support, pressure is applied at the mouth and/or
nose in spontaneously breathing patients. Continuous positive
pressure ventilation (CPAP), Non-invasive positive pressure
ventilation (NIPPV) and High flow nasal cannula (HFNC) are
examples of positive pressure non-invasive respiratory support
[2]. These modalities work by stabilizing chest wall, unloading
of diaphragm and accessory muscles of respiration, increasing
tidal volume/minute ventilation, maintaining functional residual
capacity (FRC) to prevent atelectasis and maintaining patency of
upper as well as lower airways [3]. These may also help to avoid
complications associated with invasive ventilation such as
infection, ventilator-induced lung injury, and airway edema [3].
Apart from supporting respiratory system, non-invasive
respiratory support also supports cardiovascular system [4].
Non-invasive respiratory support reduces the need for invasive
mechanical ventilation, especially in mild to moderate cases of
acute respiratory distress syndrome (ARDS) and acute lung injury
[5-7]. In LMICs, cost-effective indigenously developed CPAP
systems have been shown to reduce mortality and referral
to tertiary care neonatal intensive care units (ICUs) in term
and preterm babies with respiratory distress syndrome
[8-10]. Though pediatric critical care is well developed in
high-income countries, it still remains in its early stage
in most LMICs due to lack of well-equipped intensive care units,
trained staff, rapid access to necessary medications and
supplies. Complications and mortality from high burden diseases
like severe pneumonia, severe malaria and diarrhea can be
reduced by training healthcare providers, selecting
resource-appropriate effective indigenous equipment and
co-operation from governing bodies and industry [11]. This
review is aimed to address few issues relevant to the LMIC
settings.
Are children from LMICs with specific
respiratory problems likely to benefit from non-invasive
respiratory support?
NRS can be safely used in clinical conditions
such as pneumonia, bronchiolitis, asthma exacerbation,
post-extubation airway problems, acute respiratory failure in
immuno-compromised children, post-operative respiratory failure
(cardiac as well as non-cardiac), neuromuscular weakness, and
obstructive sleep apnea [2] (Box I). Non-invasive
respiratory support in pediatric acute respiratory failure is
associated with improvement in physiological parameters such as
heart rate, respiratory rate, saturation and decreased need for
invasive mechanical ventilation [12]. HFNC was associated with
higher ventilation free days at day 28 in children with acute
hypoxemic respiratory failure [5]. Few chart reviews and
proceedings from the Pediatric Acute Lung Injury Consensus
Conference suggest that NRS can be safely used in children with
mild to moderate- acute respiratory distress syndrome [13-15].
A recent systematic review on bubble CPAP (bCPAP) and HFNC
therapy in children (day 1 to 12 years) with severe pneumonia
and hypoxemia in developing countries concluded that bCPAP may
be effective and the use of HFNC therapy is very limited in
LMICs [16]. Non-invasive respiratory support is also commonly
used in critically ill children with congenital or acquired
heart disease with respiratory distress and was found to
decrease both intubation re-intubation rates
[17-19]. Non-invasive respiratory support is being used as first
line therapy to correct hypoxemia/hypercarbia in
immunocompromised children, especially those with mild to
moderate ARDS and stable hemodynamic status [20-22].
In the recent past, there has been a trend towards NRS use even
in obstructive lung diseases such as status asthmaticus in
children [23-25].
|
Box I Indications of Non-Invasive
Ventilation
Clinical conditions with pulmonary
shunt
Pneumonia
Acute lung injury
Inhalational injury
Pulmonary edema
Difficult intubation
Restrictive lung diseases
Scoliosis
Chest wall restriction
Interstitial lung diseases
Hypoventilation
Weaning from anesthesia
Neuromuscular disorders like spinal
muscular atrophy and
Gullian Barré syndrome
Upper airway obstruction
Obstructive sleep apnea
Altered mental status
Upper airway edema
Chronic lung disorders with
increase/retained secretions
Cystic fibrosis
Primary ciliary dyskinesia
Palliation therapy for respiratory
support
|
Non-invasive respiratory support also
has a role to support respiratory system in children with
neuro-muscular disease (NMD). In a prospective study, where
children with NMD (Duchenne muscular dystrophy, spinal muscular
atrophy, limb girdle muscular dystrophy, congenital myopathy)
and acute respiratory failure were treated with combination of
NRS and mechanical in-exsufflator during hospital stay,
physiologic indices such as PaO 2,
PCO2, pH, and PaO2/FiO2 improved
in all patients without any mortality; this highlights the role
of NRS in NMDs [26]. NRS is also commonly used in children to
prevent re-intubation during post-extubation period in high-risk
patients [27-30]. Summary of studies on utility of non-invasive
respiratory support in pediatric respiratory failure is shown in
Web Table I.
A recent systematic review on non-invasive
ventilation in children and adults in LMICs, mostly from South
Asia included 10 pediatric studies (N=1099). Pneumonia,
malaria and dengue shock syndrome were the most common
conditions requiring NRS. CPAP and bubble CPAP were commonly
used NRS modes. Pooled risk for mortality was 9.5% (95% CI
4.6-14.5) and NRS failure was seen in 10.5% (4.6-16.5). Success
rates of non-invasive respiratory support ranged from 57 to 96%
and were higher in patients with acute asthma compared to
pneumonia. Pooled risk of facial skin sores and pneumothorax
were 2.4% (95% CI 0.8-3.9) and 1.9% (95% CI 0.1-3.9),
respectively [31]. Apart from knowing the conditions where NRS
can be successful, it is also equally essential to know the
conditions where it is likely to fail and is contraindicated.
Non invasive respiratory support is likely to fail in conditions
when mean airway pressure (MAP) >11.5 cm of H 2O,
FiO2> 0.6, there is
less or minimal decrease in heart rate/respiratory rate after
1-2 hours of initiation, presence of other organ dysfunction, or
presence of severe disease (high PRISM/ Pediatric logistic organ
dysfunction scores) [32-35]. Absolute contraindications are
respiratory arrest, facial trauma/burns, upper airway
obstruction, comatose patients, intolerance, intestinal
obstruction and Gullian Barré syndrome (GBS) with absent gag
reflex. From the above discussion, we can say that common
diseases in our settings such as pneumonia, dengue, malaria are
likely to benefit from non-invasive respiratory support,
particularly in areas where ICU facilities are limited/ not
available. Complications related to NRS are: Barotrauma:
can lead to tension pneumothorax, pneumomediastinum, or massive
subcutaneous emphysema especially when the child is very
agitated; Aspiration: may occur due to gastric distension
and vomiting; Skin break down: facial skin irritation and
ulceration are seen with nasal or oronasal masks; Nasal
mucosal trauma: use of nasal masks or nasal prongs obstruct
nostrils and may lead to epistaxis in case of inadequate
humidification; Gastric distension: when inspiratory
pressures exceed lower esophageal sphincter pressure (normally
10 mmHg) or when the patient swallows air (eg, during crying),
it leads to gastric distension; Eye irritation or injury:
ocular trauma, primarily corneal abrasion or ulceration, can
occur if the edge of the mask is in contact with the eye
surface. A flow chart on initiation and monitoring of NRS is
shown in Fig.1.
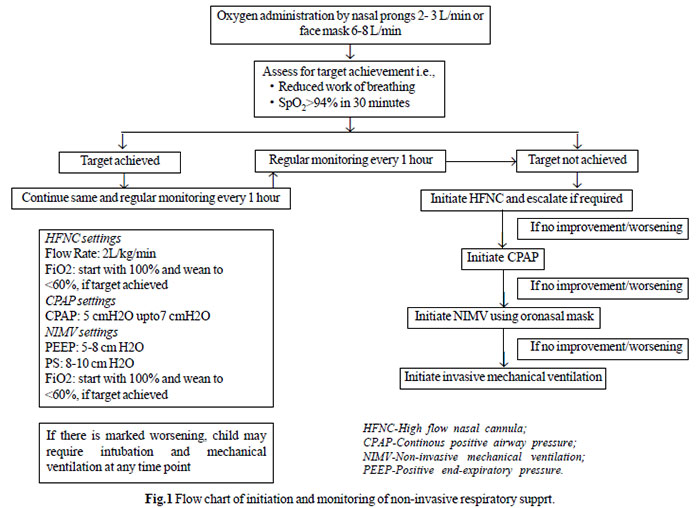 |
| |
Whether suitable indigenous equipment for
providing non-invasive respiratory support are available? If
not, is there a need to modify existing imported design of NRS
machines for their use in LMICs?
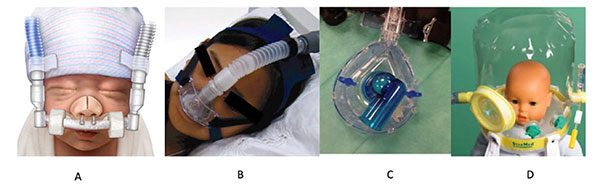
Components required for NRS are
interface, ventilator/ equipment and humidifier. Interfaces
include nasal pillow, nasal cannula, oro-nasal mask, full-face
mask, helmet (Fig. 2). In LMICs, availability and cost of
interfaces are major hurdles to provide non-invasive respiratory
support even in eligible children. Children with severe wasting
usually have less buccal pad of fat, making fit of masks
difficult. Another important equipment for non-invasive
respiratory support is ventilator/specific equipment. Classical
ICU ventilators or transport ventilators provide poor leak
compensation and need separate air and oxygen source.
Ventilators which are designed specifically for non-invasive
ventilation are usually portable, do not need separate air
source and compensate well for air leak. However, the machines
available in the market deliver minimum tidal volume of 100-150
mL which is much higher than tidal volume of infants and small
children. Another important issue to consider is the cost of
equipment. In authors’ experience, cost of portable ventilators
used for home ventilation in infants and children is
approximately INR 400 000- 500 000 (USD 5700-7200) apart from
costs of the interface (e.g., mask), ventilator circuit tubing,
humidifier, etc.; these costs may not be affordable by most
families in a LMIC. Few BiPAP ventilator machines, which are
designed for obstructive sleep apnea in adult population are
available at somewhat lower costs, may be used in older children
and adolescents. However, these machines have inherent problems
like inability to titrate FiO 2,
lack of adequate battery backup, high inspiratory time,
ineffective humidification, etc. For a PICU in a LMIC offering
invasive mechanical ventilation, it may be desirable to have
non-invasive modes in the same mechanical ventilator. In
addition, low cost HFNC and bubble CPAP equipment may also be
added. For units which do not have mechanical ventilators or
inadequate numbers of ventilators, stand-alone low-cost HFNC and
bubble CPAP equipment should be considered for installation.
 |
|
Fig. 2 Interfaces used for NIV
(a-nasal cannula; b- nasal pillow; c- oronasal mask;
d-helmet).
|
Is there a need to have innovations in
provi-ding non-invasive respiratory support in LMICs?
In LMICs, in order to overcome the
costs/availability issues, we may prepare indigenous
equipment/devices to deliver NRS. Indigenously made CPAP
equipment, bubble CPAP, have been used successfully in Indian
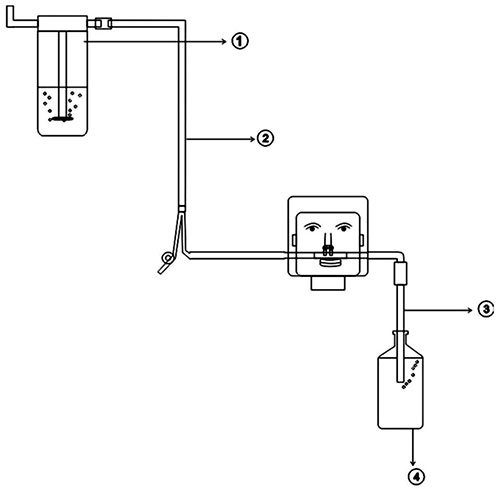
PICUs. In a retrospective study from India, 60 children with
acute hypoxic respiratory failure due to swine flu were treated
with indigenous nasal bubble CPAP (NB-CPAP) (Fig. 3),
which provided expiratory positive airway pressure of 5 cm H 2O
and delivered FiO2 of
around 70%. All patients tolerated CPAP and none required
endotracheal intubation [36].
 |
|
Fig. 3 Assembly of indigenous
CPAP.
1- Oxygen supply through flow meter; 2- Nasal cannula;
3- Intravenous tubing cut and one end is attached to
nasal cannula and other end is inserted in normal saline
bottle to exert CPAP;4- Normal saline bottle showing
bubbles during exhalation.
|
In another study from India, indigenous CPAP
was provided through flow inflating device-Jackson-Rees circuit
(JR)/Bain circuit and using face mask as interface (Fig. 4).
This study included 214 children and CPAP through flow inflating
device was successful in 89.7% of cases, of which bronchiolitis
accounted for 98.3%. A prolonged duration of CPAP support of
>96 h was required in pneumonia. CPAP failure was noted in 10.3%
of cases, the major risk factors being children <1 year and
pneumonia with septic shock [37]. Jayashree, et al. [38]
enrolled 330 children aged 1 month-12 years, with clinical
pneumonia to bCPAP group (delivered via an underwater ‘T’ tube
through nasal prongs) and nasal prongs group, and found that
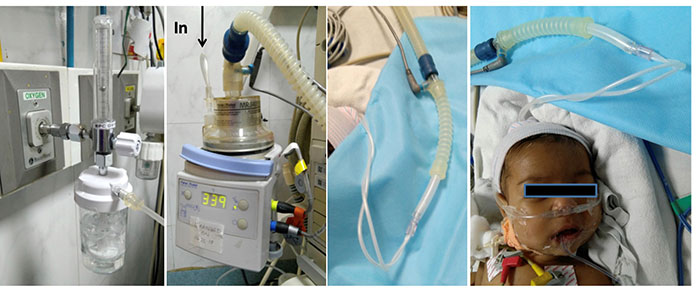
nasal CPAP is safe and effective. Indigenous HFNC circuit can
also be prepared by using O2/O2-air
mixture (blender) source, servo-control humidifier (heated wire
humidifier), corrugated tubing and nasal prongs (Fig. 5).
A blender can used to regulate FiO2. One
has to be innovative to assemble locally available equipment in
their hospitals to prepare indigenous non-invasive ventilation
equipment. However, one has to remember that quality of
indigenous equipment for NRS needs to be assessed by treating
physician.
 |
|
Fig. 4 Flow inflating bag used
for providing continuous positive airway pressure.
|
 |
|
Fig. 5 Indigenous high flow
nasal cannula; a) Oxygen source and flow meter;
b) Servo humidifier; c) connection of
nasal prongs to corrugated tubing from humidifier; d)
Nasal prongs placed in nasal cavity and should be of
appropriate size to allow leak.
|
Training healthcare professionals to provide
non-invasive respiratory support
Training of health care personnel (doctors,
nursing staff, technicians) is equally important for successful
outcome of non-invasive ventilation in intensive care. An
important aspect of training is to choose right patient at right
time for initiation. Apart from initiation, other important
aspect is to closely monitor and identify early failure within
1-2 hours of initiation and step up the respiratory support in a
timely fashion to improve outcome. In LMICs, where the nursing
staff to patient ratio is often inadequate, early
identi-fication of failure poses an important challenge. The
intensity/frequency of monitoring may actually be greater for a
child undergoing non-invasive ventilation than invasive
ventilation. So, having adequately trained man-power is critical
for safe application of non-invasive respiratory support in
critically ill children.
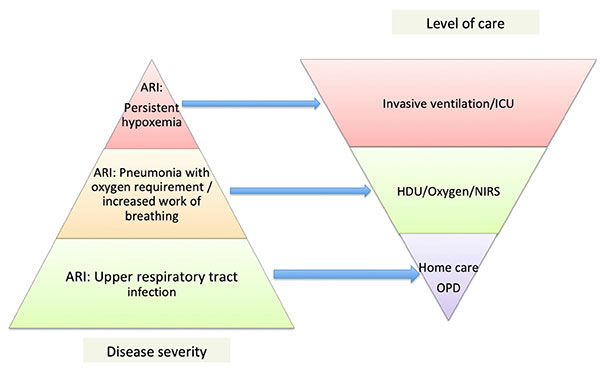 |
|
Fig. 4 Depiction of disease
severity with level of care provided. ARI-acute
respiratory infection; HDU-High dependency unit;
ICU-Intensive care unit; NRS-Non invasive
respiratory support.
|
Will non-invasive respiratory support be cost
beneficial in these countries?
A study from India [9] evaluated the cost
effectiveness of locally assembled low-cost CPAP system in
neonates with respiratory distress, and found that neonatal
mortality could be reduced using this CPAP system with cost of
only 160 INR per one CPAP system.
In another study from Malawi [8], low-cost
bubble CPAP system was used to treat neonatal respiratory
distress and led to 27% absolute improvement in the survival
when compared to standard care. A study on adults in India did
cost-effective analysis of ward-based non-invasive respiratory
support plus standard treatment with standard treatment alone in
chronic obstructive pulmonary disease (COPD) with respiratory
failure and found that ward-based NRS treatment increased the
survival of patients with COPD respiratory failure, when ICU is
not available, at a lesser cost [39]. Thus, non-invasive
respiratory support in LMICs is not only cost-effective but also
improves the outcome of patients requiring respiratory support.
Although India has now become a global market
for many biomedical equipment and established itself as
competitor for multinational counter parts, unfortunately hardly
any of the NRS equipment or their parts are manufactured in
India. So, there is an urgent need for establishing highly
effective physician-engineer-industry collaborations for
manufacturing cost effective, high quality non-invasive
equipment as good as their multi-national counter parts. Often
there are concerns about the quality of indigenous equipment;
there has to be enough efforts put in by the manufacturers to
ensure a certain level of quality of products, particularly for
the safety features.
In developing countries, a child is likely to
suffer around 0.3 episodes of pneumonia/year, and in developed
countries it is 0.03 episodes per child/year [40]. Based on
this, India is predicted to have about 700 million episodes of
acute respiratory tract infections and about 52 million episodes
of pneumonia every year [41]. For example, Broor, et al. [42]
had reported 43 episodes, 536 episodes, and 2387 episodes of
severe acute lower respiratory infections, acute lower
respiratory infections and acute upper respiratory infections,
respectively per 1000 child years from northern India. This
shows that majority of children with acute respiratory tract
infection need home based care or isolation, few children may
need hospital care and very few of them need either high
dependency unit (HDU) care or ICU care. Hence, there is a need
to invest more in development and procurement of devices
providing simple oxygen therapy or non-invasive respiratory
support as most children with acute lower respiratory tract
infection can be managed with them if intervened early and
invasive ventilation is needed only in few. A pyramid depicting
burden of respiratory illness and requirement of respiratory
support has been shown in Fig. 5. Hence, in contrast to
the usual tendency of clinicians and hospital administrations
for having more high-cost equipment for invasive mechanical
ventilation, there is a need to invest in procuring more of
non-invasive respiratory support systems for possibly a better
cost-effective solution in LMICs.
Role of non-invasive respiratory support in
COVID-19 pandemic
Children of any age can be infected with
COVID-19, but the severity seems to be less than that in adult
population. In a systematic review, children accounted for 1-5%
of total diagnosed COVID-19 cases [43]. As of April 2, 2020,
among the 1,49,760 laboratory-confirmed cases reported to the US
CDC (United States Centers for Disease Control and Prevention),
children of less than 18 years constituted only 1.7% (N=2572)
[44]. Among these children, 147 (range 5.7%-20%) were reported
to be hospitalized, with 15 (range 0.58%-2.0%) admitted to ICU.
In another report from China [45], out of 728
laboratory confirmed cases in children, 21 (2.9%) were either
severe or critically ill. Children with severe/critical disease
need respiratory support. When the respiratory status worsens in
patients with non-COVID pneumonia, physicians use non-invasive
ventilation without hesitation provided clinically appropriate.
However, when noninvasive venti-lation is considered in patients
with COVID pneumonia, there are concerns about aerosol
generation, which may cause contamination of ICU environment and
staff. There is an ongoing debate on whether to use HFNC/NIV in
patients with COVID pneumonia [46]. Appropriately fitted
interfaces in HFNC/NIV may restrict direct release of air during
expiration into the environment. However, in our set-up, limited
availability of appropriate-sized interfaces for children, lack
of negative pressure isolation rooms in all health care
facilities and limited availability of high quality personal
protective equipment to health care workers make pediatric
intensivists not to use HFNC/non-invasive respiratory support in
this scenario. Despite the apprehension associated with use of
these modalities, 137 out of 1287 ICU admitted patients (11%
[95% CI, 9%-12%]), were treated with non-invasive ventilation in
Italy [47]. In a report from China, 61 out of 84 patients
with COVID-19 ARDS received non-invasive
ventilation [48]. However, there are no data describing whether
these modalities were successful at avoiding intubation.
Hence, the decision to initiate HFNC or NIV in COVID-19 patients
should be taken by balancing the risks and benefits to the
patient, the risk of exposure to healthcare workers, and
availability of resources.
Monitoring on HFNC/NIV: If HFNC or
NIV is adminis-tered, vigilant monitoring with frequent clinical
(respiratory rates, retractions, cyanosis, sensorium) and
arterial blood gas evaluation every one to two hours is needed
to ensure efficacy and safety. Some physicians try HFNC/NIV
while the patient is in the prone position, though there is no
evidence for the same.
Precautions: Airborne precautions should
be undertaken. While using HFNC, additional surgical mask can be
placed on the patient face and lowest effective flow rate should
be used. When NIV is initiated, a full-face mask rather than a
nasal or oronasal mask is preferred to minimize particle
dispersion. The mask should have a good seal and should not have
an exit valve. For older ch ildren, helmet can be used as an
interface. Dual limb circuit with a viral filter on the
expiratory limb on routine ICU ventilator is preferred compared
to single limb circuit on portable BIPAP machines. It is
preferable to titrate ventilator setting to lowest effective
pressures (e.g., 5-10 cm H 2O).
Innovations are also being tried using a constant flow canopy
over the upper part of the patient bed, thus building a
restricted area around the patient where non-invasive
respiratory support can be safely used. This canopy system
consists of flexible plastic canopy that covers the upper part
of the body, fan filtering unit (FFU) using high efficiency
particulate air (HEPA) filters and an exhaust system creating
negative pressure and transferring the filtered air out to the
open atmosphere [48].
India has diverse health facilities and
facilities should have its own guideline whether to provide NRS
to patients with COVID-19 pneumonia depending on availability of
appropriate interfaces, personal protective equipment, negative
pressure rooms, adequate staffing, etc. We need to strike a
balance between benefit to the patient and risk to health care
workers while providing NRS.
CONCLUSION
Greater use of indigenous non-invasive
respiratory support equipment, adequate training of healthcare
providers to use and monitor and commitment from hospital
administration are important steps to improve outcomes of
children in LMICs. Though HFNC is a promising therapy, it has
not been adequately studied in LMICs and requires further
studies prior to its widespread use. Cost-effective evaluation
including assessment of optimal professional staffing levels
should be addressed in future studies of non-invasive
respiratory therapies in LMICs. To fill up the existing huge
demand supply gap of non-invasive ventilation equipment, there
is a need to develop high quality, locally manufactured,
affordable non-invasive respiratory support equipment by
facilitating partnership between governing agencies and
industry.
Contributors: KMG: literature
search, preparation of manuscript; SKK: conception of idea,
reviewed manuscript; RL: conception of idea, reviewed manuscript
and he is the corresponding author.
Funding: None; Competing
interest: None stated.
Note: Additional material related
to this study is available with the online version at
www.indianpediatrics.net
REFERENCES
1. Eichel T, Dreux ML. Negative or
positive? The Iron Lung and Poliomyelitis–Zurich,
1951. Anaesth Intensive Care. 2017; 45:13-20.
2. Zieliñska M, Zieliñski S,
Œniatkowska-Bartkowska A. Mechanical ventilation in
children–Problems and issues. Adv Clin Exp Med.
2014;23:843-8.
3. Mehta S. Noninvasive ventilation. Am
J Respir Crit Care Med.2001;163:540-77.
4. Pinsky MR. Cardiopulmonary
interactions: Physiologic basis and clinical applications.
Ann Am Thorac Soc. 2018; 15: S45-8.
5. Frat J-P, Thille AW, Mercat A, et al.
High-flow oxygen through nasal cannula in acute hypoxemic
respiratory failure. N Engl J Med. 2015;372:2185-6.
6. Yañez LJ, Yunge M, Emilfork M, et al.
A prospective, randomized, controlled trial of noninvasive
ventilation in pediatric acute respiratory failure: Pediatr
Crit Care Med. 2008; 9:484-9.
7. Ni Y-N, Luo J, Yu H, et al. The
effect of high-flow nasal cannula in reducing the mortality
and the rate of endotracheal intubation when used before
mechanical ventilation compared with conventional oxygen
therapy and noninvasive positive pressure ventilation. A
systematic review and meta-analysis. Am J Emerg Med.
2018;36:226-3.
8. Kawaza K, Machen HE, Brown J, et al.
Efficacy of a low-cost bubble CPAP system in treatment of
respiratory distress in a neonatal ward in Malawi. PLoS One.
2014; 9:e86327.
9. Daga S, Mhatre S, Borhade A, et al.
Home-made continuous positive airways pressure device may
reduce mortality in neonates with respiratory distress in
low-resource setting. J Trop Pediatr. 2014;60:343-7.
10. Hendriks H, Kirsten GF, Voss M, et
al. Is continuous positive airway pressure a feasible
treatment modality for neonates with respiratory distress
syndrome in a rural district hospital? J Trop Pediatr.
2014;60:348-51.
11. Slusher TM, Kiragu AW, Day LT, et al.
Pediatric critical care in resource-limited
settings–Overview and lessons learned. Front Pediatr.
2018;6:49.
12. Yañez LJ, Yunge M, Emilfork M, et al.
A prospective, randomized, controlled trial of noninvasive
ventilation in pediatric acute respiratory failure. Pediatr
Crit Care Med. 2008;9:484-9.
13. Fortenberry JD, Del Toro J, Jefferson
LS, Evey L, Haase D. Management of pediatric acute hypoxemic
respiratory insufficiency with bilevel positive pressure
(BiPAP) nasal mask ventilation. Chest. 1995;108:1059-64.
14. Essouri S, Chevret L, Durand P, et
al. Noninvasive positive pressure ventilation: Five years of
experience in a pediatric intensive care unit: Pediatr Crit
Care Med. 2006;7:329-34.
15. Essouri S, Carroll C. Noninvasive
support and ventilation for pediatric acute respiratory
distress syndrome: Proceedings from the Pediatric Acute Lung
Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:
S102-10.
16. Chisti MJ, Duke T, Ahmed T, et al.
The use of bubble CPAP and humidified high flow nasal
cannula oxygen therapy in children with severe pneumonia and
hypoxemia: A systematic review of the evidence. Bangladesh
Crit Care J. 2015;2:71-8.
17. Gupta P, Kuperstock JE, Hashmi S, et
al. Efficacy and predictors of success of noninvasive
ventilation for prevention of extubation failure in
critically ill children with heart disease.
PediatrCardiol 2013;34:964-77.
18. Fernández Lafever S, Toledo B, Leiva
M, et al. Non-invasive mechanical ventilation after heart
surgery in children. BMC Pulm Med. 2016;16: 167.
19. Kovacikova L, Skrak P, Dobos D,
Zahorec M. Noninvasive positive pressure ventilation in
critically ill children with cardiac disease. Pediatr
Cardiol. 2014;35:676-83.
20. Pancera CF, Hayashi M, Fregnani JH,
et al. Noninvasive Ventilation in immunocompromised
pediatric patients: Eight years of experience in a pediatric
oncology intensive care unit. J Pediatr Hematol Oncol.
2008;30:533-8.
21. Bello G, De Pascale G, Antonelli M.
Noninvasive ventilation for the immunocompromised patient:
Always appropriate? Curr Opin Crit Care. 2012;18:54-60.
22. Piastra M, De Luca D, Pietrini D, et
al. Noninvasive pressure-support ventilation in
immunocompromised children with ARDS: A feasibility study.
Intensive Care Med. 2009;35:1420-7.
23. Basnet S, Mander G, Andoh J, et al.
Safety, efficacy, and tolerability of early initiation of
noninvasive positive pressure ventilation in pediatric
patients admitted with status asthmaticus: A pilot study.
Pediatr Crit Care Med. 2012;13: 393-8.
24. Thill PJ, McGuire JK, Baden HP, et
al. Noninvasive positive-pressure ventilation in children
with lower airway obstruction: Pediatr Crit Care Med.
2004;5:337-42.
25. Pilar J, Modesto iAlapont V,
Lopez-Fernandez YM, et al. High-flow nasal cannula therapy
versus non-invasive venti-lation in children with severe
acute asthma exacerbation: An observational cohort study.
Med Intensiva. 2017;41:418-24.
26. Chen T-H, Hsu J-H, Wu J-R, et al.
Combined noninvasive ventilation and mechanical
in-exsufflator in the treatment of pediatric acute
neuromuscular respiratory failure: Combined NRS/MIE in NMD
Children With ARF. Pediatr Pulmonol. 2014;49:589-96.
27. Fioretto JR, Ribeiro CF, Carpi MF, et
al. Comparison between noninvasive mechanical ventilation
and standard oxygen therapy in children up to 3 years old
with respiratory failure after extubation: A Pilot
prospective randomized clinical study. Pediatr Crit Care
Med. 2015; 16:124-30.
28. Huang H-W, Sun X-M, Shi Z-H, et al.
Effect of High-flow nasal cannula oxygen therapy versus
conventional oxygen therapy and noninvasive ventilation on
reintubation rate in adult patients after extubation: A
Systematic review and meta-analysis of randomized controlled
trials. J Intensive Care Med. 2018;33:609-23.
29. Bonora JP, Frydman J, Retta A,
et al. Post-extubation non-invasive ventilation in the
pediatric intensive care unit: A multicenter study. Arch
Argent Pediatr. 2018;116:333-39.
30. Mayordomo-Colunga J, Alberto Medina, Corsino
Rey, et al. Non invasiveventilation after extubation in
paediatric patients: A preliminary study. BMC Pediatr.
2010;10:29.
31. Mandelzweig K, Leligdowicz A, Murthy
S, et al. Non-invasive ventilation in children and adults in
low- and low-middle income countries: A systematic review
and meta-analysis. J Crit Care. 2018;47:310-19.
32. Mayordomo-Colunga J, Medina A, Rey C,
et al. Predictive factors of non-invasive ventilation
failure in critically ill children: A prospective
epidemiological study. Intensive Care Med. 2009;35:527-36.
33. Bernet V, Hug MI, Frey B. Predictive
factors for the success of noninvasive mask ventilation in
infants and children with acute respiratory failure. Pediatr
Crit Care Med. 2005;6:660-4.
34. Piastra M, De Luca D, Marzano L, et
al. The number of failing organs predicts non-invasive
ventilation failure in children with ALI/ARDS. Intensive
Care Med. 2011;37: 1510-6.
35. James CS, Hallewell CPJ, James DPL,
et al. Predicting the success of non-invasive ventilation in
preventing intubation and re-intubation in the paediatric
intensive care unit. Intensive Care Med. 2011;37:1994-2001.
36. Kinikar A, Kulkarni R, Valvi C, et
al. Use of indigenous bubble CPAP during swine flu pandemic
in Pune, India. Indian J Pediatr. 2011;78:1216-20.
37. Anitha GF, Velmurugan L, Sangareddi
S, et al. Effectiveness of flow inflating device in
providing Continuous Positive Airway Pressure for critically
ill children in limited-resource settings: A prospective
observational study. Indian J Crit Care Med. 2016;20:441-7.
38. Jayashree M, KiranBabu HB, Singhi S,
et al. Use of nasal bubble CPAP in children with hypoxemic
clinical pneumonia-Report from a resource limited set-up. J
Trop Pediatr. 2016;62:69-74.
39. Patel SP, Pena ME, Babcock CI.
Cost-effectiveness of noninvasive ventilation for chronic
obstructive pulmonary disease-related respiratory failure in
Indian hospitals without ICU facilities. Lung India.
2015;32:549-56.
40. Rudan I, Boschi-Pinto C, Biloglav Z,
et al. Epidemiology and etiology of childhood pneumonia.
Bull World Health Organ. 2008;86:408-16.
41. Selvaraj K, Chinnakali P, Majumdar A,
et al. Acute respiratory infections among under-5 children
in India: A situational analysis. Biol Med. 2014;5:6.
42. Broor S, Parveen S, Bharaj P, et al.
A prospective three-year cohort study of the epidemiology
and virology of acute respiratory infections of children in
rural India.PLoS ONE 2007;2.
43. Ludvigsson JF. Systematic review of
COVID-19 in children shows milder cases and a better
prognosis than adults. Acta Paediatr. 2020;00:1-8.
44. CDC COVID-19 Response Team, CDC
COVID-19 Response Team, Bialek S, et al. Coronavirus disease
2019 in children– United States, February 12–April 2, 2020.
Morb Mortal Wkly Rep. 2020;69:422-26.
45. Dong Y, Mo X, Hu Y, et al.
Epidemiology of COVID-19 among children in China.
Pediatrics. 2020;145:e20200702.
46. Ñamendys-Silva SA. Respiratory
support for patients with COVID-19 infection. Lancet Respir
Med. 2020;8:e18.
47. Grasselli G, Zangrillo A, Zanella A,
et al. Baseline characteristics and outcomes of 1591
patients infected with SARS-CoV-2 admitted to ICUs of the
Lombardy region, Italy. JAMA. 2020; 323:1574-81.
48. Wu C, Chen X, Cai Y, et al. Risk
factors associated with acute respiratory distress syndrome
and death in patients with coronavirus disease 2019
pneumonia in Wuhan, China. JAMA. 2020;180:934-43.
49. AdirYachai, Segol O, Kompaniets D, et al. Covid19:
Minimising risk to healthcare workers during aerosol producing
respiratory therapy using an innovative constant flow canopy.
Eur Respir J. 2020;55:2001017.
|
|
|
 |
|

