|
clinicopathological
conference |
|
|
Indian Pediatr 2021;58:1067-1073 |
 |
Persistent Pneumonia in an Infant
|
|
Aravind Sekar, 1 Anju
Gupta,2 Amit Rawat,2
Shivaprakash M Rudramurthy,3
Rajender Kumar4, Anmol
Bhatia5
From Departments of 1Histopathology, 2Pediatrics, 3Microbiology,
4Nuclear Medicine and 5Radiodiagnosis, Postgraduate Institute of Medical
Education and Research, Chandigarh.
Correspondence to: Dr Anju Gupta, Professor, Department of Pediatrics,
PGIMER, Chandigarh. [email protected]
|
An eight month old boy presented with a subacute
febrile illness and radiological evidence of multifocal cavitatory
consolidations in the lungs. He continued to worsen despite multiple
oral and intravenous antibiotics. Preterminally, he developed
respiratory distress, hepatosplenomegaly, bicytopenia, and hepatic
dysfunction. Investigation for cause of persistent pneumonia resulted in
a diagnosis of chronic granulomatous disease on the basis of
Dihydrorhodamine assay and genetic analysis. Postmortem blood culture
grew Burkholderia cenocepacia. Autopsy revealed necrotizing
granulomatous inflammation with massive necrosis and abscesses in
bilateral lungs. No organism could be identified by traditional stains
on autopsy. Conventional PCR targeting 16S ribosomal DNA yielded
Nocardia pseudobrasiliensis. In conclusion, an unusual course of
pneumonia warrants invasive investigations for isolation of underlying
organism, which not only provides guidance to choice of antimicrobials
but also provides clue to an underlying disease.
Keywords: Autopsy, Burkholderia cenocepacia, Chronic
granulomatous disease, Nocardia pseudobrasiliensis.
|
|
Clinical protocol
History and examination: An 8 month old
boy presented with history of fever and insidious onset cough
for 1 month. He was asymptomatic till the age of 7 months when
he developed fever lasting for a week, for which he received
oral antibiotics. After an afebrile period of 1 week, he started
having intermittent episodes of fever upto 101ºF. Child had
loose stools transiently for 3 days. Subsequently, he developed
cough which worsened gradually. He had received 1 week of oral
amoxicillin-clavulanic acid and 2 weeks of intravenous
ceftriaxone and amikacin without any response, before being
referred to our centre.
He was second born to a nonconsanguineously
married couple, immunized for age, as per National immunization
schedule, with a normal development. During this admission, he
weighed 7.5 kg with length and head circumference of 79 cm and
45 cm, respectively. Vitals were stable and systemic examination
was unremarkable.
Course and management: Based on clinical
and radiological investigations, the patient was treated along
the lines of pneumonia, with presenteral meropenem and
vancomycin for 3 weeks, as the child had already received first
and second-line antibiotics earlier. In spite of persisting
fever, patient was discharged on parental request, only to be
readmitted after 5 days with worsening respiratory distress.
During the second admission, he was found to have
hepatosplenomegaly and investigations showed severe anemia,
thrombo-cytopenia, coagulopathy with very low fibrinogen and
high d-Dimer, high serum ferritin and transaminitis with
conjugated hyperbilirubinemia (Table I). In view of
persistent pneumonia, immuno-deficiency was considered and
investigations were sent accordingly (Table II). His
condition deteriorated fast and despite antibiotics, antifungals
and supportive therapy, the child died. Preterminally he
developed hypotension, hypo-glycemia and left pneumothorax. A
family history of chronic granulomatous disease (CGD) in a
paternal second-degree female cousin was elicited just prior to
demise.
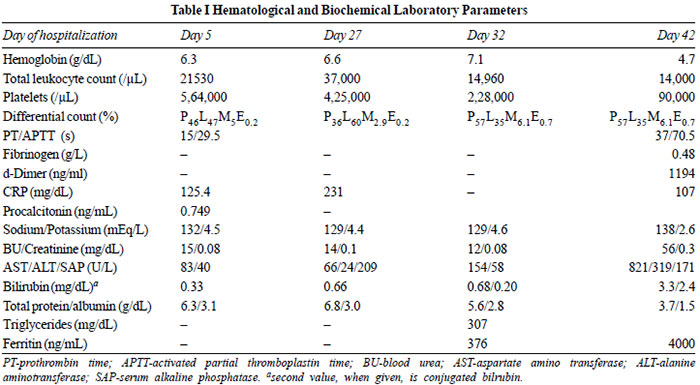
|
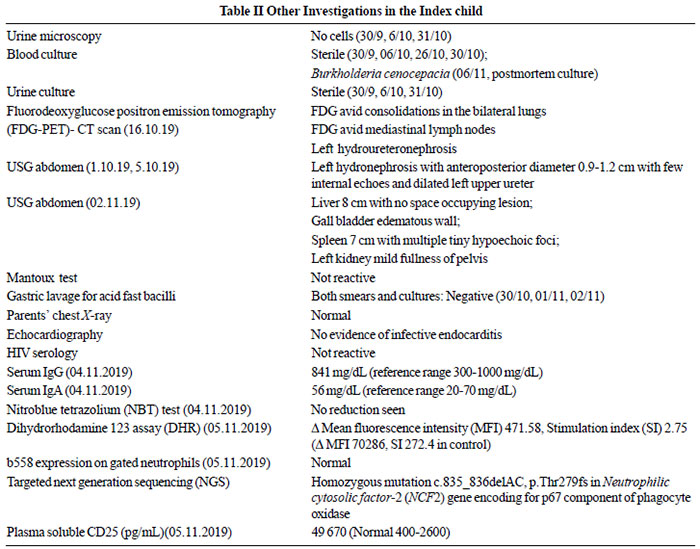 |
Unit’s final diagnosis: Burkholderia
cenocepacia sepsis with pneumonia (bacterial or fungal) with
left hydroureteronephrosis (infective or obstructive due to
granulomatous inflammation) and secondary hemo-phagocytic
lymphohistiocytosis (HLH), with underlying autosomal recessive
(AR) CGD (p67 deficiency).
Discussion
Important points of discussion in the index
child are whether CGD could have been considered in the first
admission, reason for a relatively early fatality and
explanation for the other findings such as left
hydroureteronephrosis and preterminal events.
The index child presented with fever and
insidious onset, progressive cough for 1 month. Investigations
revealed leucocytosis, thrombocytosis, sterile blood and urine
cultures, nonprogressive left hydronephrosis and radiological
evidence of consolidation in both lungs. Consolidation is
suggestive of an infectious pathology, and common bacteria
responsible for community-acquired pneumonia (CAP) at this age,
are Streptococcus pneumoniae, Staphylococcus aureus,
Moraxella catarrhalis and Hemophilus influenzae
[1]. Usually, CAP responds to antibiotics like
amoxycillin-clavulanic acid and ceftriaxone, unless complicated
with empyema or lung abscess. Since the child had an
indolent course with onset of respiratory distress two months
after onset of fever and did not respond to usual antibiotics,
CAP is unlikely and causes of persistent pneumonia need to be
considered [2]. Though Mycobacterium tuberculosis is an
important cause of persistent pneumonia, early cavitation is
extremely rare in infants [3]. This suggests possibility of
infections due to unusual organisms such as opportunistic
bacteria or fungi. Clinical manifestations and radiology both
lack specificity for underlying organism and yield of blood
culture is low at 10-30% [2]. In the absence of fine needle
aspiration cytology (FNAC) from lung lesions or bronchoalveolar
lavage, while alive, it is difficult to pinpoint etiologic
organism for persistent pneumonia.
Recurrent/persistent infections in one lobe
of lung can occur due to congenital malformations
(sequestration, bronchogenic cysts, cystic adenomatoid
malformation) and external or internal compression of airway by
lymph nodes, foreign body, or tumours. However, these were
unlikely in the index case, because he had multifocal
consolidations in both lungs. Recurrent/persistent infec-tions
in bilateral lung fields occur in a setting of congenital heart
disease, aspirations, impaired mucociliary clearance (ciliary
dyskinesia, cystic fibrosis) and immunodeficiency. While humoral
immunodeficiencies are usually associated with infections due to
community acquired bacteria, which respond to usual antibiotics,
pneumonia in cystic fibrosis, combined immunodeficiency and
phagocytic defects may be due to opportunistic pathogens [4,5].
HIV infection was ruled out in the index case.
Normal lymphocyte count rules out severe
combined immunodeficiency. As the index child had evidence of
phagocytic defect documented by no reduction in NBT test and
negligible stimulation index on DHR assay, CGD is likely.
Further investigations showed normal b558 expression ruling out
the possibility of X-linked CGD and AR-CGD due to p22
deficiency. Diagnosis was further confirmed by genetic analysis
which showed a homozygous mutation (c.835_836delAC; p.Thr279fs)
in NCF2 gene which encodes for p67 component of
phago-cytic oxidase. Thus the index child was convincingly
proven to have AR-CGD caused by p67 deficiency.
 |
|
Fig. 1 A. Chest X-ray
showing bilateral air space consolidations (right >
left); B. Chest X-ray one month later showing
progression of consolidations; C and D. PET-CT images
showing pleura based cavitating nodules.
|
The index child succumbed to the disease
during infancy. While X-linked CGD is associated with more
severe disease, severity is variable in AR-CGD due to variable
phagocyte oxidase activity [6, 7]. Severe disease in the index
child can be explained by near absent activity of phagocyte
oxidase with SI of 2.75.
Most infections in CGD are caused by catalase
positive organisms including fungi such as Aspergillus
and bacteria such as Staphylococci, Burkholderia, Serratia
and Nocardia. Enterobacteriaceae and Candida are
other important pathogens [5,6]. After introduction of
cotrimoxazole and itraconazole prophylaxis, infections with
Aspergillus, Burkholderia species and Nocardia
have been on rise [6]. Nearly 80% patients with CGD have at
least one episode of pneumonia, with Aspergillus,
Staphylococci, Burkholderia, and Nocardia
being responsible for two-thirds of the organisms [6]. In
contrast to bilateral lung involvement in the index case,
Aspergillus pneumonia in CGD typically involves one lobe
with contiguous spread to pleura, ribs and vertebrae [8].
B. cenocepacia found in post-mortem blood
culture, in the index case, is a signature organism in both
cystic fibrosis and CGD [4,6]. However, there are marked
differences between the infection pattern in cystic fibrosis and
CGD. In cystic fibrosis, this organism causes colonization of
the tracheobronchial tree [9] and can rarely cause invasive
cepacia syndrome. These patients are not able to clear the
colonized organisms and hence, spectrum of Burkholderia
species is narrow. Isolation from sputum helps in diagnosis.
In contrast, this organism causes
bronchopneumonia with central cavitation in patients with CGD
[9]. Tissue from lungs is required for isolation of organism.
Antibiotics can eradicate this organism but reinfection with
same or different Burkholderia species is common.
Invasive disease with secondary bacteremia has been described
with high mortality. The clinico-radiologic profile in the index
child is consistent with B. cenocepacia infection.
Left-sided hydroureteronephrosis, seen in
this case, could be due to granulomatous inflammation, a known
cause of obstruction of urinary tract in infants with CGD [5].
Anemia and thrombocytosis, early in the course were likely to be
multifactorial, related to both iron deficiency anemia and
chronic inflammation [5]. Preterminally, this child developed
hepatosplenomegaly, bicytopenia, coagulopathy with very low
fibrinogen and high d-Dimer, high serum ferritin and
transaminitis with conjugated hyperbilirubinemia. All these
features could be explained by secondary HLH [10], which is
further supported by the high plasma soluble CD25 levels.
Left-sided pneumo-thorax could be due to rupture of cavitating
consolidation or as a complication of ventilation.
Gastroenterologist: The diagnosis
of CGD is confirmed, though the organism causing consolidation
may be debated upon.
Pulmonologist 1: Was Mucor considered in
view of cavitatory consolidations?
Clinical discussant: Though Mucor is an
important differential in patients with cavitatory
consolidations, it is not a typical organism in CGD.
Pediatrician 1: Why Galactomannan
elevation is not common in CGD?
Clinical discussant: In CGD,
Aspergillus is locally invasive and hematogenous spread is
rare, thus making galactomannan and
b-D glucan
elevations uncommon in patients with Aspergillus
pneumonia in CGD [8].
Microbiologist 1: Galactomannan in
bronchoalveolar lavage fluid may be more sensitive than serum
galactomannan in CGD.
Pathology protocol
A partial autopsy was performed. All serous
cavities were normal.
Both lungs together weighed 220.5 g. Pleural
surface was dull and outer surface of both lungs showed multiple
nodules of varying sizes with predilection towards lower lobes.
Multiple lymph nodes measuring 0.5-1 cm were present in
pretracheal and paratracheal regions. Tracheobronchial mucosa
was congested. Cut surface of lungs showed similar nodules (Fig.
2A). Central area of large nodules showed necrosis with
abscess formation. Some nodules showed evidence of rupture of
abscess wall. Microscopy showed large irregular geographic areas
of necrosis limited by interlobar septae (Fig. 2B).
Necrosis was palisaded by dense inflammatory infiltrate rich in
epithelioid histiocytes (Fig. 2C), with well-formed
epithelioid cell granulomas, and numerous giant cells were also
noted in some places. Numerous micro-abscesses surrounded by
similar inflammatory infiltrate were observed (Fig. 2D).
No fungal profiles were identified on PAS and Groccot stains.
Gram stain and Ziehl-Neelsen stain did not reveal any organisms.
Adjoining alveolar spaces were densely infiltrated by
neutrophils and macrophages. There was evidence of diffuse acute
alveolar damage in the form of homogenous, eosinophilic hyaline
membrane along the alveolar ducts and alveoli at some places (Fig.
2E). Other areas showed proliferative phase of diffuse
alveolar damage. There was extensive fibrinous pleuritis. Lung
tissue was subjected to conventional PCR targeting 16S ribosomal
DNA region followed by Sanger sequencing. Nucleotide sequence
obtained was matched with gene bank, which revealed presence of
Nocardia pseudobrasiliensis. PCR for M. tuberculosis
and non-tubercular mycobacteria was negative.
 |
|
Fig. 2 A. Cut surface of both
lungs shows greyish white nodules of variable size with
central necrosis in larger nodules (Gross photograph);
B. Large geographic type necrosis of lung parenchyma
limited by interlobular septae (20X, H&E); C. Necrosis
is palisaded by epithelioid cell granuloma with giant
cell formation (200X, H&E); D. Abscess formation with
dense neutrophils rich inflammation in adjoining
alveolar spaces (200X, H&E); E. Glassy eosinophilic
membrane noted along alveolar wall indicating diffuse
alveolar damage (200X, H&E); F. Microscopy of liver
showing necrosis with palisading epithelioid histiocytes
(200X, H&E); G. Left ureter is dilated from
vesico-ureteric junction to renal pelvis. Cut surface of
left kidney shows mildly dilated pelvicalyceal system
(Gross photograph); H. Microgranulomas with pigmented
histiocytes are seen in the lamina propria of large
intestine (200X, H&E); I. Bone marrow shows increase in
number of histiocytes with significant hemophagocytosis
(100X, H&E).
|
Liver and spleen weighing 490 g and 186 g,
respectively, had an unremarkable capsule with mottling on the
cut surface of liver and prominent white pulp and few greyish
white lesions in cut surface of spleen. Peripancreatic and
perisplenic lymph nodes were enlarged. Microscopic examination
of liver showed preserved architecture, centrizonal hepatocyte
necrosis, dense infiltration of sinusoids by histiocytes and
micro-abscesses with central necrosis surrounded by palisading
histiocytes (Fig. 2F). Microscopic examination of spleen
showed similar abscesses. No organism could be identified by
Gram stain, Ziehl–Neelsen stain, PAS and Groccot stains.
Both kidneys weighed 121 g with unremarkable
capsule. Left ureter was grossly dilated throughout its length (Fig.
2G). Cut surface of left kidney showed minimally dilated
pelvis, the latter showing attenuated transitional lining
microscopically. No abscess or granuloma was seen in kidneys.
Tubular necrosis was seen in greyish-white lesions of left
kidney. Sections from vesicoureteric junction and urinary
bladder showed invagination of surface mucosa into lamina
propria. Lamina propria showed mild mixed inflammatory
infiltrate of histiocytes. No well-formed granulomas were seen.
Small intestine showed prominent Peyer’s
patches. Focal loss of intestinal folds was seen in large
intestine. Microscopically, granulomas were seen in lamina
propria palisaded by lymphomononuclear cells. Characteristic
pigmented histiocytes were seen in some of these granulomas (Fig.
2H). There was no evidence of cryptitis or crypt abscesses.
Peyer’s patches showed similar granuloma without necrosis.
The sinus spaces of lymph nodes were
markedly distended and infiltrated by benign histiocytes. Well-
defined granulomas without central necrosis and occasional
multinucleated giant cells were seen.
Bone marrow was hypercellular with increased
histiocytes, and hemophagocytosis of neutrophils, lymphocytes
and RBC in histiocytes (Fig. 2I). Focal hemophagocytosis
was observed in liver, spleen and lymph nodes.
Other organs such as heart, thymus, testis,
adrenal and skeletal muscles were grossly and microscopically
normal.
Final autopsy diagnosis was necrotizing
granulomatous inflammation with massive necrosis and abscesses
(N. pseudobrasiliensis), diffuse alveolar damage in the
lungs with necrotizing granulomatous inflammation and
microabscesses in liver and spleen with granulomatous colitis
with left sided hydro-ureteronephrosis, granulomatous cystitis
and granulomatous lymphadenitis. The overall pathologic features
are consistent with a diagnosis of CGD with features of shock
and HLH
Open Forum
Microbiologist 1: Nocardia
pseudobrasiliensis is usually multidrug resistant and only
cotrimoxazole may work in this infection [11,12].
Pathologist 1: What is the role of
FDG-PET in such a child?
Clinical discussant: FDG-PET is done in a
child with prolonged fever with no obvious cause on routine
investigations. The index child had evidence of bilateral
consolidation during first admission. CT scan of chest may have
served the purpose of delineating the consolidations better and
to decide on invasive investigations for microbiologic
diagnosis.
Pediatrician 2: Could the choice of
antibiotics have been different?
Clinical discussant: Empiric therapy for
infections in a patient with CGD includes staphylococcal cover
(cloxacillin or vancomycin) and cover for gram negative bacteria
(carbapenam or fluoroquinolone) [13]. Antifungal cover may be
added if the patient is sick. Change in regimen may be required
once an organism is isolated from clinical specimens [13].
Pharmacologist 1: Ceftriaxone and
cotrimoxazole could have been good choice in this child.
Gynecologist 1: What counselling was done
for the family?
Clinical discussant: Parents have been
counseled about the disease, need to investigate elder sibling
and risk of recurrence of 25% in any pregnancy. They have been
counseled regarding need of chorionic villous sampling at 9-10
weeks of gestation for prenatal diagnosis.
DISCUSSION
Microbiological identification of organism
requires invasive investigations in a child with persistent
pneumonia as clinical and radiological profiles lack etiologic
specificity and yield of blood culture is extremely low [2]. An
early FNAC from lung lesions may have altered the outcome in the
index child. Identification of organism is not only important
for appropriate antimicrobials but also gives clue regarding
underlying disease.
CGD is a prototype phagocytic defect due to
reduced phagocyte oxidase activity. Genetic defects can cause
deficiency of any of the four components namely gp91, p22, p47
and p67 of phagocyte oxidase [13]. Reduced activity of phagocyte
oxidase results in defective phagocytosis and consequent
infection with catalase positive bacteria and fungi. Pneumonia,
lymphadenitis, subcutaneus or visceral abscesses, and
osteomyelitis are frequent infections. Bacteremia and fungimea
are less frequent. Initial infection with unusual organisms such
as Burkholderia, Nocardia, Serratia and
Aspergillus should raise the suspicion [14]. Recurrent deep
staphylo-coccal infections should also warrant investigation.
Besides infections, hyperinflammation can
result in failure to thrive, hepatosplenomegaly, lymphadenopathy,
anemia, thrombocytosis, and raised inflammatory parameters [5].
Organ specific inflammation can present as colitis,
granulomatous cystitis, gastric outlet obstruction, and
hydronephrosis [5]. Diagnosis is clinched by demonstration of
reduced phagocyte oxidase activity by NBT or DHR assays [5].
Expression of b558 helps in demonstrating gp91 and p22
components of phagocyte oxidase. While X-linked CGD due to gp91
deficiency is the commonest type in the West [6], the same is
not true in other countries [7]. Owing to frequent
consanguinity, AR-CGD contributes to 50-60% of all CGD patients
in Asia. Severity of disease depends on residual activity of
phagocyte oxidase [7]. Cotrimoxazole and itraconazole
prophylaxis with or without interferon-ã have resulted in
significantly better outcomes [13]. Failure of the prophylaxis
warrants hematopoietic stem cell transplantation.
Both Burkholderia and Nocardia
are signature opportunistic organisms in CGD. Pulmonary
involvement due to Nocardia can present with focal or
multifocal consolidations with central cavitation and pleural
effusions [11,12]. Most Nocardia species are susceptible
to sulphonamides, linezolid, amikacin, imipenam, minocycline,
and moxifloxacin with Nocardia pseudobrasiliensis being
more resistant [11]. Prolonged combination therapy with 2-3
drugs is preferred for invasive disease. Steroids have been used
in combination with appropriate antibiotics in CGD patients with
Nocardia infections [15].
In conclusion, we need to be more invasive
for microbiologic diagnosis when the clinical course does not
suggest CAP. Isolation of organism not only provides guidance to
choice of antimicrobials but also provides clue to underlying
disease.
Funding: None; Competing interests:
None stated.
References
1. Skolnik N, Tien P. Community-acquired
pneumonia in infants and children. Fam Pract News.
2011;41:22.
2. Yousif TI, Elnazir B. Approach to a
child with recurrent pneumonia. Sudan J Paediatr.
2015;15:71-7.
3. Roya-Pabon CL, Perez-Velez CM.
Tuberculosis exposure, infection and disease in children: A
systematic diagnostic approach. Pneumonia. 2016;8:23.
4. Davies JC, Alton EWFW, Bush A. Cystic
fibrosis. BMJ. 2007;335:1255-9.
5. Song E, Jaishankar G, Saleh H,
Jithpratuck W, Sahni R, Krishnaswamy G. Chronic
granulomatous disease: A review of the infectious and
inflammatory complications. Clin Mol Allergy. 2011;9:10.
6. Winkelstein JA, Marino MC, Johnston
RB, et al. Chronic granulomatous disease. Report on a
national registry of 368 patients. Medicine (Baltimore).
2000;79:155-69.
7. Köker MY, Camcýoðlu Y, van Leeuwen K,
et al. Clinical, functional, and genetic characterization of
chronic granulomatous disease in 89 Turkish patients. J
Allergy Clin Immunol. 2013;132:1156-63.e5.
8. King J, Henriet S, Warris A.
Aspergillosis in chronic granulomatous disease. J Fungi.
2016;2:15.
9. Greenberg DE, Goldberg JB, Stock F,
Murray PR, Holland SM, LiPuma JJ. Recurrent burkholderia
infection in patients with chronic granulomatous disease:
11-year Experience at a large referral center. Clin Infect
Dis. 2009;48:1577-9.
10. Henter J-I, Horne A, Aricó M, et al.
HLH-2004: Diagnostic and Therapeutic Guidelines for
Hemophagocytic Lympho-histiocytosis. Pediatr Blood Cancer.
2007;48:124-31.
11. Wilson JW. Nocardiosis: updates and
clinical overview. Mayo Clin Proc.2012;87:403-7.
12. Minero MV, Marín M, Cercenado E,
Rabadán PM, Bouza E, Muñoz P. Nocardiosis at the turn of the
century: Medicine (Baltimore). 2009;88:250-61.
13. Thomsen IP, Smith MA, Holland SM,
Creech CB. A comprehensive approach to the management of
children and adults with Chronic Granulomatous Disease. J
Allergy Clin Immunol Pract. 2016;4:1082-8.
14. Marciano BE, Spalding C, Fitzgerald
A, et al. Common severe infections in Chronic Granulomatous
Disease. Clin Infect Dis Off Publ Infect Dis Soc Am.
2015;60:1176-83.
15. Freeman AF, Marciano BE, Anderson VL, Uzel G, Costas C,
Holland SM. Corticosteroids in the treatment of severe Nocardia
pneumonia in chronic granulomatous disease. Pediatr Infect Dis
J. 2011;30:806-8.
|
|
|
 |
|

