|
|
|
Indian Pediatr 2021;58: 1024-1029 |
 |
Role of Clinical Criteria and Oxygen
Saturation Monitoring in Diagnosis of Childhood Pneumonia in
Children Aged 2 to 59 Months
|
Rashmi Ranjan Das,
1
Amit Kumar Satapathy,1
Aparna Mukherjee,2
Jagdish Prasad Goyal,3
Javeed Iqbal Bhat,4
Vinod H Ratageri,5
Bhadresh Vyas,6
Rakesh Lodha,2
and ATU (Acute Respiratory Infection Treatment Unit) Group*
From Department of Pediatrics, 1AIIMS Bhubaneswar, Odisha;
2Department of Pediatrics, AIIMS New Delhi; 3Department of
Pediatrics, AIIMS Jodhpur, Rajasthan; 4 Department of
Pediatrics, Sher-i-Kashmir Institute of Medical Sciences,
Srinagar, J&K; 5 Department of Pediatrics, Karnataka
Institute of Medical Sciences, Hubli, Karnataka; 6MP Shah
Medical College, Jamnagar, Gujarat. *List of ATU Group
members provided in annexure I.
Correspondence to: Dr Rashmi Ranjan Das, Additional
Professor, Department of Pediatrics, AIIMS,
Bhubaneswar 751 019, Odisha.
Email: [email protected]
Received: April 02, 2020;
Initial review: April 11, 2020;
Accepted: December 26, 2020.
|
Background: Current WHO algorithm has
retained the signs and symptoms used in the older version
for classifying severity of childhood pneumonia.
Objective: To study the role of
clinical features (including that of current WHO criteria),
and oxygen saturation (SpO2) in the diagnosis of childhood
pneumonia.
Study design: Multicenter prospective
cohort study.
Participants: Children, 2 to 59
months of age, suffering from acute respiratory infection
(ARI).
Outcome measures: Sensitivity,
specificity, and likelihood ratios were calculated for
clinical features, and SpO2.
Results: Of a total 7026
children with ARI enrolled, 13.4% had pneumonia (37% of them
had severe pneumonia), according to WHO criteria. Based on
any abnormality on chest x ray (CXR), 46% had pneumonia. The
sensitivity and specificity of the existing WHO criteria for
diagnosis of pneumonia was 56.5% and 66.2%, respectively,
when compared against abnormalities in CXR. Cough and fever,
each had sensitivity of >80%. Audible wheeze and breathing
difficulty, each had a specificity of >80%. Sensitivity and
specificity of tachypnoea were 58.7% and 63.3%,
respectively. None of the clinical features alone had a
sensitivity and specificity of >80%. Addition of SpO2 of
<92% to chest indrawing alone or WHO criteria increased the
likelihood of diagnosis of pneumonia.
Conclusions: Current WHO criteria
based on rapid respiratory rate and/or chest indrawing has
modest sensitivity and specificity, considering CXR
abnormalities as gold standard for diagnosis of pneumonia.
Addition of SpO2 of <92% to chest indrawing alone or WHO
criteria increases the probability of pneumonia diagnosis,
and is important in the management of a child with
pneumonia.
Keywords: Acute respiratory infection,
Sensitivity, Specificity.
|
P
neumonia is a
leading cause of death in under-five children [1,2]. Over 80% children with
community acquired pneumonia (CAP) present
with cough and fever, while other features like breathing
difficulty, nausea, vomiting, and poor feeding are seen with
variable frequencies [3]. One of the major issues of CAP in
children is making a correct diagnosis.
For uniform management of childhood acute
respiratory infection (ARI) including CAP, WHO (World Health
Organization) developed an algorithm based on evidences
generated in the early 1980s [4]. The clinical criteria
adopted in this WHO algorithm used a combination of clinical
manifestations including fast breathing in a child with
cough and/or difficult breathing for diagnosing pneumonia
[4]. The sensitivity of the WHO algorithm was found to range
between 59-81% and there has been concern about its
specificity, resulting in unnecessary use of antibiotics [3,
5-11].
The WHO algorithm for pneumonia was
revised in 2014, combining severe and very severe pneumonia
as one category, with pneumonia being defined as fast
breathing and/or lower chest indrawing (LCI) [12]. However,
this revised algorithm retained the signs and symptoms used
in the older version for classifying severity of pneumonia
in children. As per a recent systematic review, absence of
cough was a significant negative predictor, while SpO2 of
£95%
or increased work of breathing (nasal flaring, grunting or
lower chest indrawing) were significant diagnostic
predictors of pneumonia [3]. There is as yet no study from
the Indian setting, assessing the diagnostic accuracy of
clinical signs and symptoms (including WHO criteria) of
pneumonia, with or without SpO2 measurement.
METHODS
This prospective, multicentric
cohort study was conducted in tertiary care teaching
hospitals in five sites in India over a 2 year period (June
2016 to May 2018). Children aged 2-59 months with ARI (any
cough and/or breathing difficulty for <2 weeks) were
enrolled. Those with chronic respiratory diseases (asthma,
cystic fibrosis, broncho-pulmonary dysplasia, airway
anomalies), congenital heart disease, gastro-oesophageal
reflux/ recurrent aspirations, immunosuppression,
radiologically confirmed pneumonia in last 2 months,
residing outside the study city, and who were critically ill
(impending respiratory failure, cyanosis at room air,
shock), were excluded. The study was initiated after
clearance by the respective Ethics Committees of all five
study sites. Children were enrolled after obtaining written,
informed consent from parents or legal guardian.
Details regarding clinical features,
nutritional and immunization status, treatment history,
demographic information, and examination findings were
recorded. A staff nurse was trained to assess breathing
difficulty by counting respiratory rate, and identifying
chest indrawing under supervision of a trained research
officer. Ausculta-tory findings were also recorded. Fever
was defined as an axillary temperature of
³37.5 ºC.
Tachypnea was defined and clinical diagnosis of pneumonia
was made as per the WHO criteria [12]. SpO2 was recorded
using Nellcor
portable pulse oximeter (measurement range 60% to 100%). As
previous studies had reported an SpO2 of <92% to indicate
pneumonia with good sensitivity and specificity, we used the
same cut-off in the present study [3,13]. Antipyretic was
given for fever and respiratory rate was reassessed after 30
minutes. In case of wheezing, salbutamol nebulization (0.15
mg/kg/dose) was adminis-tered and respiratory rate
reassessed after 10-15 minutes.
A chest X-ray (CXR) was obtained
in all children clinically assessed to have acute lower
respiratory tract infection (ALRI/pneumonia) as per the WHO
criteria. CXR was also obtained in every fifth child
assessed as no pneumonia (URI) [14]. Radiographic findings
were recorded in a standardized form based on previously
published WHO standards and definition for epidemiological
studies [15]. The digital CXR films or hard copies of CXRs
were sent to the co-ordinating center. All CXRs were read by
two independent pediatricians, who were blinded for the
clinical diagnosis of patients. In case of disagreement,
CXRs were read by a third pediatrician without knowledge of
the previous evaluations, and findings matching with
previous two were considered final. Radiographic pneumonia
was diagnosed if there was agreement on presence of any
abnormality (pulmonary infiltrate or pleural effusion) in
two independent assessments. The site investigator managed
the patient as per his interpretation based on WHO
guidelines [16].
To improvise clinical case definition CAP
with a sensitivity and specificity of 80% (sensitivity of
tachypnea with/without chest indrawing is about 69%) and
precision of 5%, a total of 256 children with pneumonia were
needed. Considering that 10% children with ARI have a
probability of pneumonia [17], 2560 children with ARI were
needed to be screened.
Statistical analysis: For
analysis, the data were entered into Microsoft excel sheet
and analyzed using Stata v.14 (Stata Corp LLC) statistical
software. Categorical data were analyzed by Chi-square test.
For studying the association between WHO pneumonia
classification and CXR findings, risk ratio (RR) with 95%
confidence interval (95% CI) was calculated. Sensitivity,
specificity, likelihood ratio (LR), and post-test
probability were calculated. A P value <0.05 was
taken as significant.
RESULTS
Out of a total 18 159 children screened
across 5 sites, 7026 children with ARI were enrolled.
According to WHO criteria, 938 (13.4%) and 6088 (86.6%) of
the enrolled children had pneumonia and no pneumonia (URI),
respectively. Severe pneumonia was diagnosed in 347/938
(36.9%) children. Baseline demographic and clinical
characteristics of the enrolled children are given in
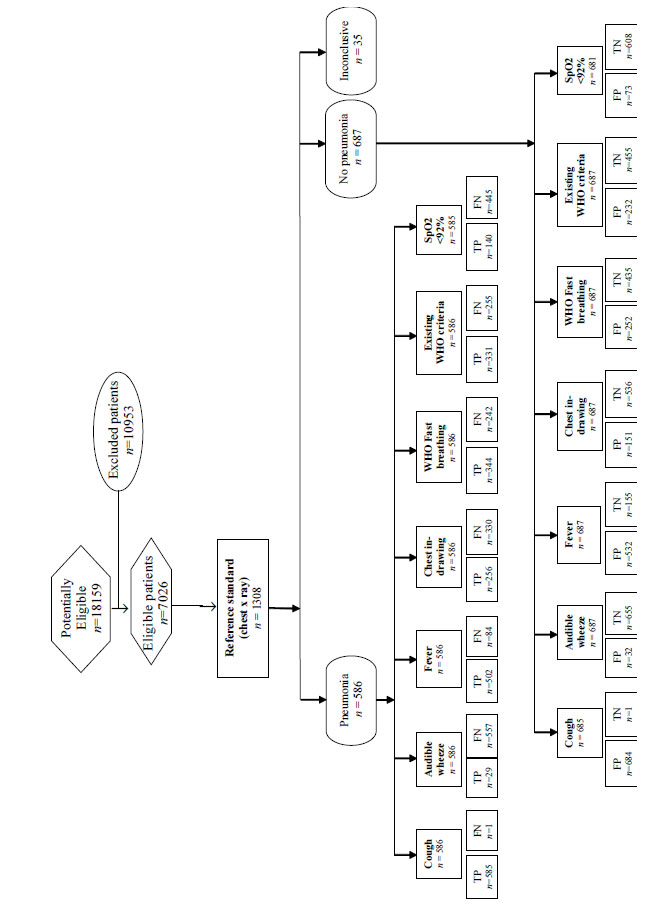
Table I. The study flow chart as per the STARD
(Standards for Reporting Diagnostic accuracy studies)
guideline is provided in Fig. 1. A total of 6,341
(90%) children were managed on ambulatory basis while 685
(10%) required hospitalization, seven of whom died.
Table I Baseline Demographic and Clinical Characteristics of Enrolled Children (N=7026)
| Characteristics |
Value |
| Age (mo) |
23 (10,40) |
| Boysa |
4251 (60.5) |
| Weight for age z-score |
-0.69 (-1.83,0.35) |
| Height/Length for age z-score |
-0.76 (-2.36,0.77) |
| Weight for height z-score |
-0.29 (-1.14,0.53) |
| Mid upper arm circumference
z-score |
-1.47 (-2.13, -0.8) |
| Cougha |
6995 (99.6) |
| Fevera |
3998 (56.9) |
|
Audible wheezea |
512 (7.3) |
|
Fast breathing post-nebulizationa |
938 (13.4) |
|
Chest indrawinga |
478 (6.8) |
|
Clinical URIa |
6021 (85.7) |
|
Clinical LRTIa |
1005 (14.3) |
|
Values in median (IQR) or ano. (%). URI/LRTI:
upper/lower respiratory tract infection. |
 |
|
Fig. 1 Study flow chart.
|
Using the rertcorded information, the
enrolled patient were re-classified, based on the WHO
criteria, and 938 (13.4%) were found to have pneumonia. Of
the 1308 CXRs available, abnormalities were reported in
those films (n=1273), which were either adequate
(features allowing confident interpretation of primary
end-point as well as other infiltrates) or suboptimal
(features allowing interpretation of primary end-point but
not of other infiltrates or findings) for reading. Rest 35
CXRs were un-interpretable. The presence of any abnormality
on CXR was considered as the gold standard for diagnosis of
pneumonia. Abnormalities in CXR were identified based on
points published by WHO: consolidation (alveolar shadows),
infiltrates (small infiltrates involving multiple segments),
interstitial shadows, and pleural effusion [15]. Around 46%
(586/1273) children had pneumonia based on these
criteria. The crude agreement between the two readers of CXR
was 80.5% (kappa=0.6, P<0.001). As shown in Table
II, a chest X-ray showing any abnormal finding,
consolidation, and alveolar infiltrates was found to be
significantly associated with a pneumonia diagnosis made as
per WHO criteria.
Table II Association Between WHO Pneumonia Classification and Chest X-Ray Findings (N=1273)
| Chest X-ray findings |
Pneumonia |
No pneumonia |
Relative risk (95% CI) |
| Any abnormal finding (n=586)a |
331 (56.5) |
255 (43.5) |
1.64 (1.45-1.85) |
| Consolidation (n=112)a |
75 (67) |
37 (33) |
2.56 (1.75-3.73) |
| Alveolar infiltrates (n=396)a |
243 (61.4) |
153 (38.6) |
2.0 (1.69-2.37) |
| Peribronchial thickening (n=104) |
52 (50) |
52 (50) |
1.26 (0.87-1.82) |
| Interstitial thickening (n=41) |
19 (46.3) |
22 (53.7) |
1.09 (0.6-1.99) |
| Atelectasis (n=5) |
3 (60) |
2 (40) |
1.89 (0.32-11.28) |
|
All values expressed as n (%). aP<0.001. WHO: World
Health Organization.
|
The diagnostic accuracy of clinical
parameters and SpO2 for pneumonia is detailed in
Web
Table I. Neither cough nor wheeze had a significant LR
for ruling in or ruling out the diagnosis of pneumonia. The
parameters like breathing difficulty, fast breathing, chest
indrawing, existing WHO criteria for pneumonia, SpO2 <92%,
existing WHO criteria + SpO2 <92%, existing WHO criteria
and/or SpO2 <92%, chest indrawing + SpO2 <92%, existing WHO
criteria present and SpO2 <92% applied serially had a
significant positive LR as well as negative LR (except
fever, which had a significant negative LR only). Positive
LR among confirmed pneumonia cases ranged from 1.5 (for
breathing difficulty) to 2.7 times (for chest indrawing +
SpO2 <92%) in confirmed pneumonia cases compared to those
without. Negative LR ranged from 0.85 (for chest indrawing +
SpO2 <92%) to 0.64 (for fever, and existing WHO criteria
and/or SpO2 <92%, both) in those with pneumonia compared to
those without. The prevalence (pre-test probability) of
pneumonia in the present study was 46%. We calculated the
post-test probability for parameters having a LR+ of
³2.0
and/or a LR– of £0.5.
Addition of a SpO2 of <92% increased the post-test
probability of diagnosing pneumonia to 66% in case of
existing WHO criteria, and 69% in case of chest indrawing.
DISCUSSION
In this multi-center hospital-based
observational study across five sites in India, none
of the clinical parameters (either single or in combination)
had a sensitivity and specificity of >80% for diagnosis of
childhood pneu-monia. The overall analysis suggests that,
the current WHO criteria for pneumonia have modest
sensitivity (56.5%) and specificity (66.2%), which is in
agreement with the findings of a previous meta-analysis [8].
The prevalence of radiological pneumonia
in the present study was 46%, similar to previous studies
[13]. Primary end point pneumonia is usually defined as
presence of consolidation or pleural effusion with or
without other infiltrates (e.g., interstitial
infiltrates/thickening, atelectasis, peri-bronchial
thickening, and alveolar infiltrates not sufficient to refer
as a consolidation) [8]. Other infiltrates are commonly seen
in viral or atypical pneumonia. In the present study, only
consolidation and alveolar infiltrates were found to be
significantly associated with WHO pneumonia, which probably
means that majority had bacterial pneumonia [13], which is
consistent with the finding of a relatively high proportion
of severe pneumonia cases, in the present study (37%) [18].
As per the WHO algorithm, fast
breathing/tachypnea is an important indicator of childhood
pneumonia, and studies from developed countries also support
this [19,20]. In the present study; however, the WHO defined
fast breathing had a sensitivity of 58.7% and specificity of
63.3%. In a study from Mexico, WHO defined tachypnea as a
sole clinical sign had 74% sensitivity and 67% specificity
for the diagnosis of radiological pneu-monia [19]. The
sensitivity was reduced, and specificity was increased (84%)
when other clinical signs were combined. An additional
observation in this study was that, in children with
pneumonia of <3 days’ duration, tachypnea had a sensitivity
and specificity of 55% and 64%, respectively. In a recent
systematic review, presence of tachypnea (respiratory rate
>40 breaths/min) in children beyond infancy, was not
strongly associated with pneumonia diagnosis [3]. It is
important to note that, absence of tachypnea does not rule
out the diagnosis of pneumonia. in children under-five years
of age [8,20].
Fever, which is commonly seen in
pneumonia [21, 22], had a sensitivity of 85.7% and
specificity of 26.6% for diagnosing pneumonia in the present
study. The British Thoracic Society (BTS) Guideline mentions
that, in children below 3 years, high fever along with chest
indrawing and tachypnea (>50/min) is suggestive of pneumonia
[22]. On the contrary, a systematic review showed that
temperature >37.5°C was not strongly diagnostic of pneumonia
[3]. Chest signs on auscultation (e.g., crackles, rales, or
rhonchi) were neither sensitive nor specific for pneumonia
[3].
A LR+ of
³2.0 and
a LR £0.5
has been shown to change the post-test probability of
disease appreciably. In the present study, neither cough nor
audible wheeze (7.3% children) has a significant LR for
ruling in or ruling out pneumonia diagnosis. This is an
interesting observation, as cough has been the most sought
symptom of pneumonia. A recent systematic review [3] found
that none of the features including cough, audible wheeze,
poor feeding, breathing difficulty, or duration of illness
>3 days had a significant likelihood for diagnosing
pneumonia, though absence of cough had a significant
negative LR (LR 0.47; 95% CI 0.24 to 0.70) in ruling out the
diagnosis of pneumonia. Also, SpO2
£95% and
increased work of breathing (nasal flaring, grunting or
lower chest indrawing) (LR+ 2.1) had a significant
likelihood to diagnose pneumonia. Studies using other cutoff
SpO2 values
(i.e., 96%, 92%, and 90%) had lower LR+, whereas, SpO2 >96%
had a LR– of 0.47 [3,23]. The poor diagnostic performance of
auscultatory findings (e.g., presence of wheeze or crackles)
could be because these are subjective parameters. The
present study shows that the probability of having pneumonia
improved to 66% among those tested positive for WHO criteria
with a SpO2 of <92%, and to 69% among those with chest
in-drawing and a SpO2 of <92%. None of the parameters in the
present study were found to have negative LR of
£0.5,
thus making them inappropriate for ruling out the pneumonia
diagnosis. Our findings are different from previously
published studies [3,8], probably because of variation in
the age of included children (only few studies included
children >5 years age), geographical location (e.g., high
altitude, urban/rural), care-seeking behavior, duration of
disease, and prevalence of malnutrition.
The limitation of the present study is
that we could not carry out subgroup analysis of factors
like age, duration of symptoms at presentation and severity,
which are known to modify the diagnostic performances in a
previously published study [19].
To conclude, current WHO criteria
based on rapid respiratory rate and/or chest in-drawing has
modest sensitivity and specificity, taking CXR abnormalities
as gold standard for diagnosis of pneumonia. Addition of
SpO2 of <92% to chest indrawing alone or to WHO criteria
increases the probability of pneumonia diagnosis, and is
important in the management of a child with pneumonia.
Acknowledgements: AIIMS
Bhubaneswar: Ms Jyotshnarani Sahoo and Ms Manaswini
Biswal; AIIMS Jodhpur: Mr Vikas Patwa; KIMS Hubli:
Dr Prakash Wari (HOD Pediatrics), Vedasree and Gayatri;
SKIMS Srinagar: Umaisa Zehra and Saba.
Ethical clearance: The study
was approved by Institutional ethics committee of all the
six study sites.
Contributors: RRD: involved in
protocol development, supervision of study, data collection
and analysis, and manuscript writing. AKS: involved in data
collection, management of patients, and manuscript writing.
AM, RL: involved in protocol development, data analysis, and
manuscript writing. JPG, JIB, VHR, BV: involved in protocol
development, and manuscript writing. All the authors have
approved the manuscript version to be published.
Funding: This work was supported by
Bill and Melinda Gates Foundation through The INCLEN Trust
International (Grant number: OPP1084307). The funding source
had no contribution in study design, implementation,
collection and interpretation of data and report writing.
Competing interest: None stated.
Annexure I
*Members of The ATU (Acute Respiratory
Infection Treatment Unit) Group
Partha Sarathi Ray, AIIMS, Bhubaneswar, Odisha;
Kana Ram Jat, AIIMS, New Delhi; Bashir Ahmad Charoo,
SKIMS, Srinagar, J&K; Daisy Khera, Prawin Kumar and
Deepak Singhal, AIIMS, Jodhpur, Rajasthan; Samarendra
Mahapatro, AIIMS, Bhubaneswar, Odisha; Kuldeep Singh,
AIIMS, Jodhpur, Rajasthan; Sushil K Kabra, AIIMS,
New Delhi.
|
What is Already Known?
• Current WHO case definition
based on rapid respiratory rate and/or chest
in-drawing has modest sensitivity and specificity
considering CXR abnormalities as gold standard for
diagnosis of childhood pneumonia.
• Addition of SpO2 <92% to
chest indrawing alone or to WHO criteria increases
probability of diagnosing pneumonia.
|
REFERENCES
1. McAllister DA, Liu L, Shi T, et
al. Global, regional, and national estimates of
pneumonia morbidity and mortality in children younger
than 5 years between 2000 and 2015: A systematic
analysis. Lancet Glob Health. 2019;7:e47-57.
2. Fadel SA, Boschi-Pinto C, Yu S, et
al. Trends in cause-specific mortality among children
aged 5-14 years from 2005 to 2016 in India, China,
Brazil, and Mexico: An analysis of nationally
representative mortality studies. Lancet.
2019;393:1119-27.
3. Shah SN, Bachur RG, Simel DL,
Neuman MI. Does This child have pneumonia? The rational
clinical examination systematic review. JAMA.
2017;318:462-71.
4. World Health Organization (WHO).
The management of acute respiratory infections in
children In: Practical guidelines for outpatient care.
Geneva: WHO, 1995.
5. Sazawal S, Black RE, Pneumonia
Case Management Trials Group. Effect of pneumonia case
management on mortality in neonates, infants, and
preschool children: a meta-analysis of community-based
trials. Lancet Infect Dis. 2003;3:547-56.
6. Hazir T, Qazi SA, Bin Nisar Y, et
al. Comparison of standard versus double dose of
amoxicillin in the treatment of non-severe pneumonia in
children aged 2-59 months: a multi-centre, double blind,
randomized controlled trial in Pakistan. Arch Dis Child.
2007;92:291-7.
7. Harari M, Shann F, Spooner V,
Meisner S, Carney M, de Campo J. Clinical signs of
pneumonia in children. Lancet. 1991;338:928-30.
8. Rambaud-Althaus C, Althaus F,
Genton B, D’Acremont V. Clinical features for diagnosis
of pneumonia in children younger than 5 years: a
systematic review and meta-analysis. Lancet Infect Dis.
2015;15:439-50.
9. Mulholland EK, Simoes EA, Costales
MO, McGrath EJ, Manalac EM, Gove S. Standardized
diagnosis of pneu-monia in developing countries. Pediatr
Infect Dis J. 1992; 11:77-81.
10. Singhi S, Dhawan A, Kataria S,
Walia BN. Validity of clinical signs for the
identification of pneumonia in children. Ann Trop
Paediatr. 1994;14:53-8.
11. Redd SC, Vreuls R, Metsing M,
Mohobane PH, Patrick E, Moteetee M. Clinical signs of
pneumonia in children attending a hospital outpatient
department in Lesotho. Bull World Health Organ.
1994;72:113-8.
12. Falade AG, Tschäppeler H,
Greenwood BM, Mulholland EK. Use of simple clinical
signs to predict pneumonia in young Gambian children:
the influence of malnutrition. Bull World Health Organ.
1995;73:299-304.
13. Awasthi S, Rastogi T, Mishra N,
et al. Chest radiograph findings in children aged 2-59
months hospitalised with community-acquired pneumonia,
prior to the introduction of pneumococcal conjugate
vaccine in India: a prospective multisite observational
study. BMJ Open. 2020;10: e034066.
14. World Health Organization (WHO).
Revised WHO classification and treatment of childhood
pneumonia at health facilities, 2014. Accessed on 29
August, 2019. Available at:https://www.who.int/maternal_child_
adolescent/ documents/child-pneumonia-treatment/en
15. Dowell SF, Schwartz B, Phillips
WR. Appropriate use of antibiotics for URIs in children:
Part I. Otitis media and acute sinusitis. The Pediatric
URI Consensus Team. Am Fam Physician.
1998;58:1113-8,1123.
16. Cherian T, Mulholland EK, Carlin
JB, et al. Standardized interpretation of paediatric
chest radiographs for the diagnosis of pneumonia in
epidemiological studies. Bull World Health Organ.
2005;83:353-9.
17. Mathew JL, Patwari AK, Gupta P,
et al. Acute respiratory infection and pneumonia in
India: a systematic review of literature for advocacy
and action: UNICEF-PHFI series on newborn and child
health, India. Indian Pediatr. 2011;48:191-218.
18. Rudan I, Boschi-Pinto C, Biloglav
Z, Mulholland K, Campbell H. Epidemiology and etiology
of childhood pneumonia. Bull World Health Organ.
2008;86:408-16.
19. Palafox M, Guiscafre H, Reyes
H, Munoz O, Martinez H. Diagnostic value of tachypnoea
in pneumonia defined radiologically. Arch Dis
Child. 2000;82:41-5.
20. Leventhal J. Clinical predictors
of pneumonia as a guide to ordering chest
roentgenograms. Clin Pediatr. 1982;21: 730-4.
21. Campbell H, Byass P, Lamont AC,
et al. Assessment of clinical criteria for
identification of severe acute lower respiratory tract
infections in children. Lancet. 1989;1:297-9.
22. British Thoracic Society. British
Thoracic Society Guidelines for the Management of
Community Acquired Pneumonia in Childhood. Thorax.
2002;57:i1-24.
23. Mahabee-Gittens EM, Grupp-Phelan
J, Brody AS, et al. Identifying children with pneumonia
in the emergency department. Clin Pediatr (Phila).
2005;44:427-35.
|
|
|
 |
|

