|
|
|
Indian Pediatr 2020;57: 1040-1048 |
 |
Indian Academy of Pediatrics Position Paper
on Kawasaki Disease
|
|
Bhaskar Shenoy, 1
Surjit Singh,2
M
Zulfikar Ahmed,3 Priyankar Pal,4
Suma Balan,5 Vijay Viswanathan,6
Sagar
Bhattad,7 Anand P Rao,8
Maitri Chaudhuri,9 Digant D Shastri10
and Santosh T Soans11
From Departments of 1Pediatrics, Manipal
Hospitals, Bangalore, Karnataka; 2Advanced Pediatric Centre,
Post Graduate Institute of Medical Education and Research (PGIMER),
Chandigarh; 3Department of Cardiology, Pushpagiri Medical
College, Tiruvalla, Kerala; 4Department of Pediatric
Rheumatology Institute of Child Health, Kolkata, West Bengal; 5Department
of Rheumatology, Amrita Institute of Medical Sciences, Kochi, Kerala;
6Jupiter Hospital, Thane, Maharashtra; 7Aster CMI
Hospital, Bangalore, Karnataka; 8Manipal Hospitals, Indira
Gandhi Institute of Child Health, Bangalore, Karnataka; 9Department
of Cardiology, Manipal Hospital, Bangalore, Karnataka; 10Killol
Children Hospital, Surat, Gujarat; and 11AJ Institute of
Medical Sciences, Mangalore, Karnataka; India.
Correspondence to: Dr Bhaskar Shenoy, Head,
Department of Pediatrics, Manipal Hospitals, Bangalore, Karnataka,
India. Email: bshenoy@gmail.com
Published online: August 28, 2020;
PII: S097475591600188
|
|
Objective: To formulate
practice guidelines on diagnosis and management of Kawasaki
disease (KD) for Indian children. Justification: KD is a
systemic vasculitis that predominantly affects infants and
children less than 5 years of age. Coronary artery abnormalities
(CAA) develop in around 15-25% of untreated children with KD.
Coronary artery involvement can lead to long-term cardiovascular
implications such as development of premature coronary artery
disease. Diagnosis of KD is essentially clinical based on
recognition of a constellation of characteristic symptoms and
signs. Timely diagnosis and initiation of intravenous
immunoglobulin (IVIG) therapy is known to produce five-fold
reduction in the incidence of CAA. As there is no confirmatory
laboratory test for KD, the diagnosis may be missed if one is
not familiar with the nuances of clinical diagnosis. Process:
A committee was formed under the auspices of Indian Academy of
Pediatrics in early 2018 for preparing guidelines on KD in
Indian children. A meeting of the consultative committee was
held in Mumbai, and a draft protocol was devised. All members
scrutinized the recent publications on the subject and an
attempt was made to arrive at a broad consensus. Published
guidelines on the subject were also reviewed.
Recommendations: The diagnosis is clinical and is aided by
laboratory and 2D echocardiography. First line of therapy is
IVIG, and should be started expeditiously once the diagnosis is
made.
Keywords: Coronary artery
abnormalities, Diagnosis, Intravenous Immunoglobulin, Infliximab,
Management.
|
|
K awasaki Disease (KD) is an
acute febrile illness that commonly affects children below 5 years of
age. Classified under pre-dominantly medium vasculitides, it has a
predilection to involve coronary arteries. Ever since the first report
by Dr. Tomisaku Kawasaki from Japan in 1967 [1], the disease has been
increasingly reported world-wide. KD has become one of the leading
causes of acquired heart disease among children in many developed
countries.
Incidence of KD has been increasing significantly
over the last decade, even in India, possibly due to a combination of an
actual increase in incidence and also due to heightened awareness
amongst the pediatricians [2]. A high index of suspicion supported with
relevant laboratory tests and imaging (2D echocardiogram) is often
needed in establishing the diagnosis. Though various consensus
guidelines are available for diagnosis and management of KD, a
nation-wide consensus for a resource constrained setting like ours is
the need of the hour.
PROCESS
A National Consultative Group was constituted under
the auspices of Indian Academy of Pediatrics (IAP) in March, 2018 for
preparing the guidelines on KD in Indian children. This group of experts
consisted of pediatricians, pediatric rheumatologists and pediatric
cardiologists known for their expertise and experience in treating KD
across the country. A meeting of the consultative committee was held in
Mumbai in March, 2018 to discuss the scientific contents. The members
reviewed the available literature and discussed various aspects of
forming the guidelines and a draft protocol was devised. This was
reviewed by all the members and a final draft recommendation was formed
through a virtual meeting. The draft recommendations formulated by the
group were circulated among the members and a consensus document was
finalized.
DIAGNOSIS
We have two established criteria that could be used
as a guide for diagnosis of KD The American Heart Association (AHA)
criteria [1] and the Japanese criteria [7]. AHA criteria have been
discussed in this document and are detailed in Box I.
|
Box I Classical Diagnostic Clinical Criteria
of Kawasaki Disease by the American Heart Association [1]
Fever persisting >/=5 days
History/Presence of >/=4 principal features
Changes in extremities (pedal edema in
acute phase, periungual peeling in sub acute phase
Polymorphous rash
Bilateral bulbar conjunctival injection
without exudates
Changes in lips and oral cavity
Cervical lymphadenopathy (>1.5 cms
diameter)
Exclusion of other diseases with similar
findings.
All manifestations may not be present at the same time in a
given child, as they are often transient. However, a thorough
history is likely to elicit findings which maybe presently
absent.
|
Clinical Features
Thorough history and assessment of clinical findings
play a major role in the diagnosis, as there are no specific tests.
Principal Clinical Findings
Diagnosis of KD is usually made on the basis of fever
for ³5 days
along with the history/presence of ³4
out of the 5 key clinical features. Diagnosis is made as per features
given in Box I but the presence of classic clinical
presentation or coronary artery abnormality, the diagnosis of KD can be
made in less than 5 days.
Fever: The most common manifestation is fever,
which is often high grade and remittent type. If untreated, fever
continues for 1-3 weeks and resolves spontaneously by 3 to 4 weeks, mean
duration of fever being 11 days.
Conjunctival injection: Bilateral, painless and
non-exudative conjunctival injection with peri-limbal sparing usually
begins in first few days after fever onset, seen in 80-90% cases. Slit
lamp examination might reveal anterior uveitis during the first week of
fever. Purulent conjunctivitis should suggest alternate diagnosis.
Oral changes: Bleeding, crusting, dryness,
erythema and fissuring of lips are common mucosal changes noted in KD
patients. Oral mucosal and pharyngeal erythema can also be seen.
Erythema of tongue along with the presence of prominent papillae results
in a strawberry tongue appearance.
Cervical lymphadenopathy: Cervical adenopathy is
usually non-specific and the least common clinical finding. Unilateral
enlargement of a cervical node >1.5
cm diameter in the anterior triangle of neck may be noted. Occasionally
the lymph node mimics suppurative lymphadenitis and may be associated
with retropharyngeal/parapharyngeal edema (phlegmon) mimicking a
retropharyngeal abscess on MRI. But presence of associated clinical
features of KD helps in clinching the diagnosis.
Rash: A maculopapular erythematous rash that
begins in trunk, later extending to extremities and face, is usually
seen by 5 days of onset of the illness. Sometimes it resembles a
scarlatiniform, erythroderma, erythema multiforme, or urticaria like
rash. Bullous, vesicular or petechial rashes are usually not seen and
suggests an alternate diagnosis.
Extremity changes: During the acute phase,
erythema of palms and soles along with edema and induration of hands and
feet may be seen. Desquamation of fingers and toes usually occurs 10-20
days after the onset of fever and typically starts in the periungual
region. It may extend to involve the entire palm and sole.
Other Clinical Findings
Perianal or perineal desquamation is typically
seen during the acute phase of KD, as early as day 6 of fever and is a
useful clinical pointer.
Reactivation of BCG scar: Erythema and induration
can occur at the site of BCG scar. Though noted in a small proportion of
children with KD, it is virtually patho-gnomonic when other findings are
missing [1].
Nervous system: Irritability is a common finding
especially marked in infants. It is usually out of proportion to the
degree of fever and thought to be a manifestation of aseptic meningitis.
Profound sensorineural hearing loss may be present. Facial palsy, though
rare, has been well documented. Prolonged unexplained fever with extreme
irritability may be the only clinical manifestation in many infants
below 6 months of age without any of the principal clinical signs of KD.
Gastrointestinal system: Diarrhea, vomiting, pain
abdomen, hepatitis, pancreatitis and gallbladder hydrops can be present.
Genitourinary system: Urethritis/meatitis is a
common feature in the acute phase presenting as sterile pyuria. Less
common features are hydrocele and phimosis.
Musculoskeletal system: Pain and swelling of
inter-phalangeal joints may occur during the acute phase. Arthritis of
large joints (knees and ankles) usually occur during the convalescent
phase and is seen in 10-15% of cases.
Respiratory system: Tachypnea, dyspnea,
and cough may rarely be seen. Chest radiograph may reveal peri-bronchial
or interstitial infiltrates.
Cardiovascular: Pericarditis, myocarditis,
valvular dys-function, congestive heart failure, and peripheral gang-rene
are the cardiovascular manifestations of KD.
About 5% of children may present with cardio-vascular
collapse and shock that may be difficult to differentiate from toxic
shock [8,9]. High index of suspicion and presence of accessory clinical
features helps in clinching the diagnosis. KD shock is readily
responsive to IVIg which helps in differentiating from a viral
myocarditis.
Beau lines: Transverse grooves in the
nails can be noted 1-2 months after the onset of illness indicating a
catabolic process in the preceding weeks.
Definitions used in KD diagnosis are provided in
Box II, and approach to a child with suspected incomplete KD
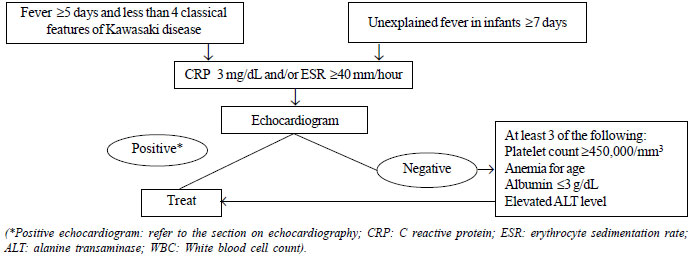
is shown in Fig. 1.
|
Box II Definitions Used in Diagnosis of
Kawasaki Disease
Complete KD: Patients with fever
of at least 5-day duration with presence/history of 4 or more of
the 5 principal clinical findings are labelled as typical or
classic KD.
Incomplete KD: Presence of fever with
less than 4 out of the 5 principal clinical criteria with
compatible laboratory or echocardiography findings suggest
incomplete KD. Often seen in infants
<6
months and children >6 years of age, the incomplete clinical
picture often delays the diagnosis. Approach to a child with
suspected incomplete KD is shown (Fig. 1).
Atypical KD: Patients who along with the
usual clinical features of KD also have few unusual clinical
manifestations like pulmonary involvement, renal impairment are
diagnosed to have atypical KD.
The terms atypical KD and incomplete KD
are inter-changeably used, but recent consensus is to use
atypical KD in patients who have unusual clinical features and
complications of KD.
|
 |
|
Fig. 1 Evaluation of suspected
incomplete Kawasaki disease (Source AHA 2017) [1].
|
Laboratory Tests
Diagnosis of KD is about pattern recognition with
impetus being on a good history and detailed physical examination.
Laboratory tests are non-specific and are only supportive and laboratory
findings vary with the course of illness.
Hemoglobin: Mild to moderate normocytic, normo-chromic
anemia is common.
Leucocyte count: Leukocytosis is usually seen in
acute phase of illness with neutrophilic predominance.
Platelet count: Thrombocytosis is one of the
significant lab findings in KD. Platelet count starts rising after first
week, reaching a peak in the third week and normalizing by 4-6 weeks.
Thrombocytopenia is uncommon but can occur in first week.
Thrombocytopenia is a risk factor for development of CAA and may be a
marker of incipient macrophage activation syndrome [10,11].
Acute phase reactants like Erythrocyte
sedimentation rate (ESR) and C-reactive protein (CRP) are almost always
elevated in KD. IVIG therapy by itself can cause an elevation in ESR
leading to doubts in the mind of the treating physician. Hence,
CRP is more useful to assess response to treatment with IVIG. Macrophage
activation syndrome which can rarely complicate KD should be suspected
in patients with severe clinical disease associated with minimally
elevated ESR and markedly elevated CRP. It might be prudent to look for
an elevated serum ferritin to confirm this suspicion.
Serum transaminases: Mild to moderate elevation
is seen in around 50% of patients.
Serum albumin: Hypoalbuminemia is often noted in
the acute phase suggesting severe inflammatory process.
Sterile pyuria (>0 cells/high power field with
sterile cultures): This is due to urethritis and sometimes be
mistaken for urinary tract infection in infants.
Procalcitonin levels are usually normal, but elevated
levels are associated with increased risk of IVIg resistance and CAA
[12]. Serum Pro-BNP (Pro-brain natriuretic peptide) and N terminal Pro
BNP (NT-ProBNP) levels are elevated in KD and can serve as useful
biomarkers in distinguishing incomplete KD and closely mimicking febrile
illnesses. Serum levels of NT-Pro-BNP > 225 pg/mL can assist in the
diagnosis of KD (suggesting myocardial dysfunction) (86.5% sensitivity
and 94.8% specificity) [13]. ECG may reveal evidence of myocarditis and
conduction disturbances. An ultrasound of the abdomen may show
hepatomegaly, hepatosplenomegaly, acalculous cholecystitis (gall bladder
hydrops).
Echocardiography
Echocardiography is the imaging modality of choice
for diagnosis, risk stratification, treatment planning, prog-nostication
and follow-up of any suspected or confirmed KD. KD is a clinical
diagnosis and role of echocardio-graphy is to only confirm/exclude
cardiac involvement, especially coronary arteritis. Thus, treatment of
KD should not be withheld for local non-availability of pediatric
cardiologist. Simultaneously, the pediatrician should refer to the
pediatric cardiologist if pyrexia of unknown origin lasts longer than 7
days.
Objectives of Echocardiography in KD
To confirm the diagnosis in case of suspected
incomplete KD, though a normal echocardiogram does not exclude the
diagnosis.
To quantify coronary changes in proven KD.
To look for other cardiac complications like
myocarditis and cardiovascular collapse (5%), valvular
regurgi-tation (e.g., mitral regurgitation), pericardial
effusion [1,8,9].
To assess response to therapy by serial
echocardio-graphy (regression, persistence or progression of
aneurysm, myocarditis and valvular dysfunction).
To look for myocardial ischemia secondary to
coronary involvement, usually seen in giant/large aneurysms.
Rarely rupture of aneurysm with cardiac
tamponade especially in acute phase with rapid enlargement of
aneurysm.
Prognostication and counselling of family.
Long term follow-up of KD with persistent CAA.
Echocardiographic Changes in KD
The cardiac involvement in KD can be grouped into (a)
early changes (b) subacute changes (c) late changes.
(a) Early changes (1st week of fever):
Coronary changes are uncommon in the first week. The
important clues are myocarditis (prevalence 50-70%), pericarditis, small
pericardial effusion and transient mild to moderate mitral regurgitation
(23-27%). We recommend use of advanced echo modalities like myocardial
performance index and tissue doppler to document myocarditis in addition
to standard parameters like ejection fraction (EF) and fractional
shortening (FS) [14,15].
7% of children with KD in US present with
cardio-vascular collapse (KD shock syndrome). The unique features of KD
myocarditis are (1) it presents early (2) precedes coronary arteritis,
(3) transient and resolves earlier than other causes of myocarditis as
inflammation and myocardial edema subside. In doubtful cases, serum NT
pro BNP may be used as a surrogate marker, although it is nonspecific
and cut off values yet to be clearly defined [13,16,17]. We reiterate
that normal coronaries in the first week do not exclude KD.
(b) Subacute changes (after 1st week of fever):
The highlight of this phase is detection of coronary involvement and
its aftermath.
Some tips and clues for successful echo in KD child
are given in Box III. The coronary involvement as per z
score classification is as follows [1]: No involvement: z score
always <2; and Dilatation only: 2 to < 2.5. Aneurysms as per size: Small
CAA: ³2.5 to
<5 mm; Medium CAA: ³5
to <10 mm and absolute dimension >8 mm; Large/Giant CAA:
³10 mm or absolute
dimension ³8
mm. Aneurysms as per shape: saccular or fusiform.
Table I Differential Diagnoses of Kawasaki Disease (KD) and Differentiating Features
| |
KD |
Scarlet fever |
Measles |
SJS |
TSS |
SJIA |
| Strawberry tongue |
Present |
Present |
Absent |
Absent |
Absent |
Absent |
| Red eyes |
Present (non- |
Absent |
Exudative |
Absent |
Exudative |
Absent |
|
exudative) |
|
conjunctivitis |
|
conjunctivitis |
|
| Red lips |
Present |
Absent |
Absent |
Absent |
Absent |
Absent |
| Response to antibiotics |
Does not respond |
Brisk response in 48 h |
NR |
NR |
NR |
NR |
| Peeling |
Perineal and periungual |
Generalized |
NR |
NR |
Generalized |
NR |
| Follicular tonsillitis |
Usually absent |
May be present |
NR |
NR |
NR |
NR |
| Edema of extremities |
Present |
Absent |
Absent |
Absent |
Absent |
Absent |
| Koplik spots |
Absent |
NR |
Present |
NR |
NR |
NR |
| Oral ulcers |
Absent |
NR |
NR |
Present |
NR |
NR |
| Hepatosplenomegaly |
Absent |
Absent |
NR |
NR |
NR |
Present |
| Hypotension /renal impairment |
Absent |
NR |
NR |
NR |
Present |
NR |
| Leukocyte counts |
Elevated |
May be elevated |
Normal |
NR |
NR |
Elevated |
| ESR and C-reactive protein |
Elevated |
May be normal |
NR |
NR |
NR |
Elevated |
|
Box III Tips for Successful Echocardiography in a Child With
Suspected Kawasaki Disease
Sedation should be used, as these children
(especially infantile KD) are extremely irritable and toxic.
To accurately identify coronary arteries,
we recommend use of highest frequency echo transducers (10-12
Hz).
The main coronary segments to be visualized
are: left main coronary artery (LMCA) bifurcating into left
anterior descending artery (LAD) and circumflex (Cx), right
coronary artery (origin, mid and distal segments).
The luminal diameter from inner edge
to edge is taken in zoomed mode. Please note all measurements
are to be compared with the child's body surface area. Weight
and especially height are to be considered while interpreting
coronary sizes. Z Scores are then calculated as per BSA.
|
The Heart Beyond the Coronaries
Apart from early phase, echo during the subacute and
long term phases should focus also on:
Aortic root dilatation and aortopathy
Cardiac valves: Late onset regurgitation
is attributed to fixed damage to valve apparatus by the inflammatory
mechanism.
Myocardial function: Both global and
regional wall motion abnormalities (RWMA) perfused by particular
coronary territories are to be reported. Abnormal RWMA is a clue of
myocardial ischemia and prompts further analysis by CT or direct
coronary angiography.
How Frequently Should One Repeat Echo in a Child with
KD?
At diagnosis.
Uncomplicated patients: 1-2 weeks and
also 4-6 weeks after treatment. This is because dilatation is
unusual beyond 6 weeks. Normal coronaries may be discharged from
cardiology care after 12 months but the medical records should
permanently mention the diagnosis of KD.
For significant and evolving coronary
abnormalities: At least twice per week till luminal dimensions
stabilize and we should look specifically for thrombus. After that
at 2 weeks, 4-6 weeks, 3 months and then every 6-12 months till
parameters normalize.
To detect coronary artery thrombosis it may be
reasonable to perform echocardiography for patients with thrombus at
diagnosis, expanding large or giant aneurysms twice per week while
dimensions are expanding rapidly and at least once weekly in the
first 45 days of illness, and then monthly until the third month
after illness onset, as failure to escalate thromboprophylaxis is a
primary cause of morbidity and mortality.
Long Term Cardiac Assessment in KD
Long-term status is when the patient is stable after
the acute illness and the coronary artery luminal dimensions are not
increasing or progressing (usually within 15 to 45 days).
5% of acute coronary syndrome in US has been
attributed to missed KD in childhood [18,19].
Normal coronaries at initial presentation
usually have no long term sequelae.
Small or moderate aneurysms usually demonstrate
normalization of luminal dimensions, infrequently stenosis may
happen. Development of late aneurysms especially with coexistent
stenosis is also reported especially with repeat KD or suboptimal
initial treatment.
Coronary artery events (thrombosis, stenosis,
intervention, MI, death) occurred in 1% of those with an aneurysm Z
score <10 and an absolute dimension <8 mm, in 29% of those with a Z
score ³10
but an absolute dimension <8 mm, and in 48% of those with both a Z
score ³10
and an absolute dimension ³8
mm [20, 21].
Subclinical functional impairment (fibrofatty
changes, necrotic core and calcification) of these coronaries have
been observed with advent of intravascular ultrasound (IVUS) and
optical coherence tomography (OCT). Interestingly wall thickening
was found more in those coronaries where aneurysms normalized on
longitudinal follow up. PET scan shows increased uptake in these
areas [22-24]. Clinically these translate to impaired myocardial
flow and reduced response to traditional coronary vasodilators like
nitroglycerin. This poses a risk to myocardial infarction in KD
survivors.
Limitations of echocardiography: Despite its
primary position as a diagnostic modality for KD, echocardiography has
some limitation:
Abnormal coronaries are seen in only 20- 25% of
KD. Hence, a normal echo does not preclude KD [1].
Coronary artery aneurysms usually appear after
1st week. It must be repeated in all KD patients after 2 and 6 weeks
[1].
Cardiac sequelae in classical and incomplete KD
are same. So, cardiologist has to be more meticulous while imaging
suspected atypical KD because diagnosis rests on 2 D echo and
laboratory findings.
Role of Other Cardiovascular Imaging Modalities
Acute phase :
Echocardiography is the best modality.
Medium and long term phase: As the child
grows, transthoracic echocardiography may not be able to visualize
especially the distal coronary segments. Apparent normalization of
coronary diameters may also be due to intimal calcification and
fibrofatty changes. So, use of CT coronary angiography, PET scanning,
cardiac MRI and documenting inducible myocardial ischemia (Dobutamine
stress echocardio-graphy, stress thallium scan, PET) to assess myocar-dial
function and ischemia in older children, adolescents and adult survivors
is recommended. Exercise TMT alone is not sufficient to detect these
changes. If any of these are positive, direct coronary angiography as a
planner for subsequent angioplasty or bypass surgery is to be done.
Differential Diagnosis
Infections :
Bacterial
(streptococcal, leptospirosis, rickettsia), Viral (measles, adenovirus,
Epstein Barr virus).
Toxin related: Staphylococcal scalded skin
syndrome, toxic epidermal necrolysis.
Inflammatory: Systemic juvenile idiopathic
arthritis.
Drug hypersensitivity: Steven-Johnson syndrome,
drug reaction with eosinophilia and systemic symptoms (DRESS), mercury
hypersensitivity.
Gastrointestinal features like paralytic ileus, gall
bladder hydrops, greenish diarrhea, jaundice and raised transaminases
may mimic other gastrointestinal infections or surgical conditions.
Sterile pyuria and CSF pleocytosis can masquerade as urinary tract
infection or aseptic meningitis.
A fever that does not appear to respond to
antimicrobials should always raise the consideration of alternate
pathologies like inflammatory or vasculitic illness like KD.
TREATMENT
Acute Kawasaki Disease
The goal of treatment is to control the acute
inflammation and prevent long term coronary sequelae. IVIG and high-dose
aspirin are the cornerstones in the management of KD, although the role
of high-dose aspirin in the acute stages is debatable. Treatment should
be initiated promptly and must not be delayed awaiting echocardiography,
when the clinical features are suggestive of KD.
Single dose of IVIG 2g/kg administered over 12-24
hours should be given within 10 days of illness, preferably in the first
7 days [1]. Timely administration of IVIG reduces the development of
CAAs from 15-25 to 3-5%, and the risk of giant aneurysms to 1% [1].
IVIG should be considered even in patients with >10
days of illness with persistent fever, systemic inflammation evidenced
by elevated ESR or CRP (>3.0 mg/L), or presence of CAAs. IVIG may not be
needed in patients who had resolution of fever with normal inflammatory
parameters and normal echocardiography findings [25].
Dose of aspirin used in the acute stages is 30-50
mg/kg/day in 3-4 divided doses, that is continued until the patient is
afebrile for 48 hours. The dose of aspirin (ASA) is reduced to 3-5
mg/kg/day and continued for 6-8 weeks and stopped if CAAs are not
detected in the 6th week echocardiography. The anti-platelet dose of
aspirin is continued in patients who have persistent CAAs until the
normalization of coronary artery dimensions. Patients on long-term
aspirin need influenza vaccination yearly to reduce the risk of Reye
syndrome.
Multiple studies have come up recently, demons-trating
the beneficial use of corticosteroids along with IVIG in children
predicted to have an increased risk of CAAs and IVIG resistance [3].
Addition of glucocorti-coids (prednisolone) to IVIg has been shown to
reduce the risk of CAAs, duration of fever, and inflammation in Japanese
children who are at a high risk for resistance to IVIG therapy. A
recently published Cochrane database systemic review has even suggested
that a long course of steroids along with IVIG should be considered in
all children with KD until further evidence are available [26].
Recommended use of steroids in KD: Oral
prednisolone (2 mg/kg/day) to be initiated with IVIG and gradually
tapered over 15 days after normalization of CRP levels.
In IVIG responsive patients, fever usually subsides
by 36-48 hours along with decrease in inflammatory parameters. Patients
with recurrent KD, defined as a repeat episode of KD after complete
resolution of the first episode, should receive standard therapy with
IVIG and ASA.
Anticoagulation in Kawasaki disease is indicated in
the following situations: (a) Giant aneurysm, multiple or complex
aneurysms, presence of thrombus; (b) associated stenosis; and (c)
peripheral gangrene.
It is prudent to initiate with LMW heparin followed
by oral warfarin to maintain INR of 2-2.5. However in view of the
difficulty of maintaining the target INR in children on oral
anticoagulants, one may consider continuing long term thromboprophylaxis
with LMW heparin only after proper parental counselling.
For arterial thrombosis/peripheral gangrene-
thrombolytic therapy has been tried in addition to anticoagulation.
Treatment of incomplete KD: Incomplete forms
should be treated in the same manner as complete KD.
Resistant Kawasaki Disease
Children who have persistence or recurrence of fever
36 hours after the end of IVIG infusion are considered to be IVIG
resistant [1]. Around 10 to 20% of patients are IVIG resistant [27].
Prolonged fever and unresponsiveness to the first dose of IVIG are
significant risk factors for CAAs.
Risk scores for predicting non response to IVIG:
Egami [28], Sano [29] and Kobayashi [30] scoring systems are some of the
scoring systems that have been shown to predict IVIG resistance.
There is no established consensus on the
pharmacologic treatment of refractory KD. Various therapeutic options
available -
IVIG retreatment: Many experts recommend
retreatment with second dose of IVIG 2g/kg. Rate of refractoriness to
the second dose IVIG is around 22-49% [31].
Corticosteroids: Furukawa, et al. [32]
compared the effectiveness of second dose IVIG and IV prednisolone in
patients with IVIG resistant KD. They found that incidence of CAA and
treatment failure were similar between 2 groups; however, the steroid
group had a faster defervescence of fever and improvement in
inflammatory markers [32]. The AHA recommends that a short duration of
high-dose glucocorticoids could be a reasonable treatment option in
patients with IVIG resistant KD [1].
Infliximab: Infliximab is a chimeric monoclonal
anti TNF- a
antibody. Dose is 5 mg/kg given intravenously over 2 hours. Studies have
not demonstrated superiority of infliximab over others in IVIG-resistant
KD in terms of coronary artery outcomes though fever and other
constitutional features resolve well. The AHA recommends the use of
infliximab as a substitute for a 2nd dose IVIG or steroids in resistant
KD [33,34].
Cyclosporine: Cyclosporine inhibits lymphocyte
activation by blocking the NFAT-calcineurin pathway that is thought to
influence disease susceptibility and development of CAAs in KD [35]. The
AHA recommends the use of cyclosporine as a possible third or
fourth-line therapy in patients with KD.
Plasma exchange: Used rarely for children who
have active inflammation despite multiple doses of IVIG,
corticosteroids, and infliximab.
Cytotoxic agents: Cyclophosphamide is used to
treat other severe vasculitides, but the risks of cytotoxic agents
limits its use.
Statins: Statins, hydroxymethylglutaryl coenzyme
A-reductase inhibitors, have been shown to reduce cholesterol levels as
well as improve surrogate markers of atherosclerosis and cardiovascular
disease. Huang, et al. [36] reported a beneficial effect of
short-term (3 months) statin treatment (simvastatin, 10 mg/day as a
single dose at bed time) in KD patients complicated with CAL. Chronic
vascular inflammation is also significantly improved, as well as
endothelial dysfunction, with no adverse effects. However, long-term and
randomized control trials are needed before further conclusions can be
made.
It has been recently reported that atorvastatin is
able to inhibit critical steps (T cell activation and proliferation,
production of the pro-inflammatory cytokine TNF-a,
and up-regulation of matrix metalloproteinase-9 and an elastolytic
protease) known to be important in the development of coronary aneurysms
in an animal model of KD, suggesting that statins may have therapeutic
benefits in KD patients [37]. Taken together, statins may be beneficial
as an adjuvant therapy in KD patients with CAL.
Management of Cardiovascular Sequelae
Coronary artery aneurysm is a potential serious
cardiac complication of KD. With giant coronary artery aneurysm, there
is increased risk of thrombosis, stenosis, ischemia, infarction and
death [38,39]. The goals of long-term management are to prevent
thrombosis and myo-cardial ischemia while maintaining optimal
cardiovascular health [39].
Medical therapy for myocardial protection:
a- blockers used are
carvedilol, metoprolol or bisoprolol. They decrease the risk of
myocardial infarction and death by reducing myocardial oxygen demand.
ACE inhibitors or ARBs also protect against myocardial infarction and
death. Statins in addition to their cholesterol lowering action have
other pleiotropic effects in inflammation, endothelial dysfunction,
oxidative stress, platelet aggregation, coagulation and fibrinolysis,
which make them useful in the management of KD [37].
Thromboprophylaxis: Antiplatelet drugs like
aspirin are commonly used in KD. In giant aneurysm or large distal
aneurysms, a dual antiplatelet treatment with aspirin and clopidogrel is
preferred. Anticoagulation with warfarin to achieve a target INR of 2-3
is used. LMWH is equally effective to warfarin, used in young children
in whom dosing with warfarin is difficult [1].
Surgical management: is rarely required in
pediatric age group. It includes percutaneous coronary intervention or
coronary artery bypass grafting [38].
Macrophage activation syndrome (MAS) is a dreaded
complication that may rarely occur characterized by persistent fever,
pancytopenia, liver dysfunction, hepato-splenomegaly, hyper-ferritinemia,
hypofibrino-genemia, elevated serum lactate dehydrogenase, and
hypertrigly-ceridemia. Prompt treatment with pulse methylpredni-solone
along with IVIg may result in favorable outcome [1].
KD should be diagnosed and treated by primary care
pediatricians. However, involvement of a pediatric rheumatologist is
required in some circumstances:
Incomplete/atypical KD,
KD in infancy,
presence of CAL at diagnosis,
IVIG-resistant KD,
KD shock syndrome, and
suspicion of a macrophage activation syndrome.
CONCLUSION
Kawasaki disease is the most common cause of acquired
heart disease in children in the developed world. It is being
increasingly recognized and treated in various parts of our country.
Pediatricians must be aware of the varied manifestations of KD. Early
diagnosis and prompt treatment can result in better outcomes.
REFERENCES
1. McCrindle BW, Rowley AH, Newburger JW, et al.
Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A
Scientific Statement for Health Professionals from the American Heart
Association. Circu-lation. 2017;135:e927-99.
2. Singh S, Vignesh P, Burgner D. The epidemiology of
Kawasaki disease: A global update. Arch Dis Child. 2015; 100:1084-88.
3. Burns JC. History of the worldwide emergence of
Kawasaki disease. Int J Rheum Dis. 2018;21:13-5.
4. Sano T, Makino N, Aoyama Y, et al. Temporal
and geographical clustering of Kawasaki disease in Japan:2007-2012.
Pediatr Int. 2016;58:1140-45.
5. Singh S, Aulakh R, Bhalla AK, et al. Is
Kawasaki disease incidence rising in Chandigarh, North India? Arch Dis
Child. 2011;96:137-40.
6. Singh S, Bhattad S. Kawasaki disease incidence at
Chandigarh, North India, during 2009-2014. Rheumatol Int.
2016;36:1391-97.
7. Diagnostic Guidelines of Kawasaki Disease. Japan
Kawasaki Disease Research Center, Japan Kawasaki Disease Research
Committee [Internet] Tokyo: Japan Kawasaki Disease Research Center,
Japan Kawasaki Disease Research Committee; c2012. [cited 2001 Jan 9].
8. Gatterre P, Oualha M, Dupic L, et al.
Kawasaki disease: An unexpected etiology of shock and multiple organ
dysfunction syndrome. Intensive Care Med. 2012;38: 872-78.
9. Kanegaye JT, Wilder MS, Molkara D, et al.
Recognition of a Kawasaki disease shock syndrome. Pediatrics.
2009;123:783-89.
10. Nofech-Mozes Y, Garty B-Z. Thrombocytopenia in
Kawasaki disease: A risk factor for the development of coronary artery
aneurysms. Pediatr Hematol Oncol. 2003; 20:597-601.
11. Garcνa-Pavσn S, Yamazaki-Nakashimada MA, Bαez M,
Borjas-Aguilar K, Murata C. Kawasaki disease complicated with macrophage
activation syndrome: A systematic review. J Pediatr Hematol Oncol.
2017;39:445-51.
12. Shao S, Luo C, Zhou K, et al. Predictive
value of serum procalcitonin for both initial and repeated
immunoglobulin resistance in Kawasaki disease: a prospective cohort
study. Pediatr Rheumatol. 2019;17:78.
13. Dahdah N, Fournier A. Natriuretic peptides in
Kawasaki Disease: The myocardial perspective. Diagnostics (Basel).
2013;3:1-12.
14. Yellen ES, Gauvreau K, Takahashi M, et al.
Performance of 2004 American Heart Association recommendations for
treatment of Kawasaki disease. Pediatrics. 2010;125: e234-e41.
15. Heuclin T, Dubos F, Hue V, et al.
Increased detection rate of Kawasaki disease using new diagnostic
algorithm, including early use of echocardio-graphy. J Pediatr.
2009;155:695-99.
16. Lin KH, Chang SS, Yu CW, et al. Usefulness
of natriuretic peptide for the diagnosis of Kawasaki disease: A
systematic review and meta-analysis. BMJ Open. 2015;5:e006703.
17. Dahdah N, Siles A, Fournier A, et al.
Natriuretic peptide as an adjunctive diagnostic test in the acute phase
of Kawasaki disease. Pediatr Cardiol. 2009;30:810-17.
18. Burns JC, Shike H, Gordon JB, Malhotra A,
Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents
and young adults. J Am Coll Cardiol. 1996; 28:253-57.
19. Daniels LB, Tjajadi MS, Walford HH, et al.
Prevalence of Kawasaki disease in young adults with suspected myocardial
ischemia. Circulation. 2012;125:2447-53.
20. OGara PT, Kushner FG, Ascheim DD, et al.
2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial
Infarction: A Report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice Guidelines.
Circulation. 2013;127:e362-e425.
21. Akagi T, Rose V, Benson LN, Newman A, Freedom RM.
Outcome of coronary artery aneurysms after Kawasaki disease. J Pediatr.
1992;121:689694.
22. Furuyama H, Odagawa Y, Katoh C, et al.
Assessment of coronary function in children with a history of Kawasaki
disease using 15O-water positron emission tomography. Circulation.
2002;105: 2878-84.
23. Muzik O, Paridon SM, Singh TP, Morrow WR,
Dayanikli F, Di Carli MF. Quantification of myocardial blood flow and
flow reserve in children with a history of Kawasaki disease and normal
coronary arteries using positron emission tomography. J Am Coll Cardiol.
1996;28:757-62.
24. Suda K, Tahara N, Honda A, et al. Statin
reduces persistent coronary arterial inflammation evaluated by serial 18
fluorodeoxyglucose positron emission tomography imaging long after
Kawasaki disease. Int J Cardiol. 2015;179:61-2.
25. Burns JC. Frequently asked questions regarding
treatment of Kawasaki disease. Glob Cardiol Sci Pract. 2017:30.
26. Wardle AJ, Connolly GM, Seager MJ, Tulloh RM.
Cortico-steroids for the treatment of Kawasaki disease in children.
Cochrane Database Syst Rev. 2017;1:CD011188.
27. Tremoulet AH, Best BM, Song S, et al.
Resistance to intravenous immunoglobulin in children with Kawasaki
disease. J Pediatr. 2008; 153:117-21.
28. Egami K, Muta H, Ishii M, et al.
Prediction of resistance to intravenous immunoglobulin treatment in
patients with Kawasaki disease. J Pediatr. 2006;149:237-40.
29. Sano T, Kurotobi S, Matsuzaki K, et al.
Prediction of non-responsiveness to standard high-dose gamma-globulin
therapy in patients with acute Kawasaki disease before starting initial
treatment. Eur J Pediatr. 2007;166:131-37.
30. Kobayashi T, Inoue Y, Takeuchi K, et al.
Prediction of intravenous immunoglobulin unresponsiveness in patients
with Kawasaki disease. Circulation. 2006;113:2606-612.
31. Burns JC, Capparelli EV, Brown JA, Newburger JW,
Glode MP. Intravenous gamma-globulin treatment and retreatment in
Kawasaki disease.US/Canadian Kawasaki Syndrome Study Group. Pediatr
Infect Dis J. 1998;17: 1144-48.
32. Furukawa T, Kishiro M, Akimoto K, Nagata S,
Shimizu T, Yamashiro Y. Effects of steroid pulse therapy on
immunoglobulin-resistant Kawasaki disease. Arch Dis Child.
2008;93:142-46.
33. Masuda H, Kobayashi T, Hachiya A, et al.
Infliximab for the treatment of refractory Kawasaki disease: A
nationwide survey in Japan. J Pediatr. 2018;195:115-20.
34. Son MB, Gauvreau K, Burns JC, et al.
Infliximab for intravenous immunoglobulin resistance in Kawasaki
disease: A retrospective study. J Pediatr. 2011;158: 644-49.
35. Suzuki H, Terai M, Hamada H, et al.
Cyclosporin A treatment for Kawasaki disease refractory to initial and
additional intravenous immunoglobulin. Pediatr Infect Dis J.
2011;30:871-76.
36. Huang SM, Weng KP, Chang JS, Lee WY, Huang SH,
Hsieh KS. Effects of statin therapy in children complicated with
coronary arterial abnormality late after Kawasaki disease: A pilot
study. Circ J. 2008;72:1583-87.
37. Blankier S, McCrindle BW, Ito S, Yeung RS. The
role of atorvastatin in regulating the immune response leading to
vascular damage in a model of Kawasaki disease. Clin Exp Immunol.
2011;164:193-201.
38. JCS Joint Working Group. Guidelines for Diagnosis
and Management of Cardiovascular Sequelae in Kawasaki Disease (JCS
2013). Circulation. 2014;78: 2521-62.
39. Suda K, Iemura M, Nishiono H, et al. Long-term prognosis
of patients with Kawasaki disease complicated by giant coronary
aneurysms: A single-institution experience. Circulation. 2011;
123:1836-42.
|
|
|
 |
|

