|
|
|
Indian Pediatr 2014;51:
875-879 |
 |
Effect of Inclusion of Hepatitis B Vaccine in
Childhood Immunization Program in India:
A Retrospective Cohort Study
|
|
Rakesh Aggarwal, JJ Babu,*R Hemalatha,*Anumulu
Venkateshar Reddy,
*Divyanshu Sharma and Tarun Kumar
From the Department of Gastroenterology, Sanjay
Gandhi Postgraduate Institute of Medical Sciences, Lucknow; and
*National Institute of Nutrition, Hyderabad, India.
Correspondence to: Dr Rakesh Aggarwal, Department of
Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical
Sciences, Lucknow 226 014, India.
Received: January 31, 2014;
Initial review: February 18, 2014;
Accepted: September 15, 2014.
|
Objective: To assess the effectiveness of hepatitis B
immunization program in a field setting in India.
Design: Serological survey
of retrospective cohorts of children, vaccinated or not vaccinated with
hepatitis B vaccine.
Setting: Rural field areas
of five districts in Andhra Pradesh state, where childhood hepatitis B
immunization began in 2003.
Participants: Children
aged 5-11 years who had received HB immunization (n=2674; 1357
boys) or not received such immunization (n=2350; 1236 boys).
Main Outcome Measures:
Serum HBsAg, anti-HBc and anti-HBs (quantitative) using automated
enzyme-immunoassays in the year 2010.
Results: Anti-HBs
positivity was higher among immunized than in unimmunized children (53%
vs.18%; P<0.001), and anti-HBc positivity was lower (1.1%
vs 10.8%: P<0.01). HBsAg positivity was low in both the
groups (0.15% and 0.17%; P=0.855). Anti-HBs positivity rate
declined with increasing age.
Conclusions:
Administration of hepatitis B vaccine as part of Universal immunization
program led to anti-HBs in a large proportion of children and a
reduction in anti-HBc positivity, a marker of hepatatis B virus
infection. These data provide evidence supporting efficacy of hepatitis
B immunization program in an Indian field setting, justifying the
decision to include it in the universal immunization program.
Keywords: Evaluation, Impact, Program,
Vaccination.
|
|
Administration of hepatitis B vaccine to all
newborns has been recommended in order to prevent chronic hepatitis B
virus (HBV) infection, and the associated disease burden [1-4].
Inclusion of this vaccine in universal infant immunization programs in
countries where HBV infection is highly endemic has led to a marked
reduction in prevalence of chronic HBV infection, and in rate of
occurrence of hepatocellular cancer [5,6].
In India, 2-4% of healthy population has chronic HBV
infection [7]. Government of India has included hepatitis B vaccine in
the National universal immunization program in the entire country in
2011-12 [8]. Initially, a 3-dose schedule of 6, 10 and 14 weeks or of 0,
6 and 14 weeks (if birth dose could be given) was used; this was later
changed to 6, 10 and 14 weeks with an additional dose being given within
24 hours of birth, where possible.
Effectiveness of HB vaccine in field situations is
assessed using prevalence rates of: (i) hepatitis B surface
antigen (HBsAg), an indicator of chronic HBV infection, (ii)
anti-HBc, a marker of number of HBV infections, whether cleared or
persistent, and (iii) anti-HBs, a protective antibody [9]. With
successful vaccination, the prevalence of HBsAg and anti-HBc is expected
to decline and that of anti-HBs to rise. The impact of infant
immunization programs on HBsAg and anti-HBc prevalence is best measured
when the immunized cohort reaches 5-7 years of age, since HBV infections
acquired before this age have a high propensity to become chronic, and
those acquired later rarely do so; thus majority of chronic HBV
infections that could occur have accumulated by this age.
Though data on coverage and drop-out rates of
hepatitis B vaccine in the Indian setting are available [8], no data are
yet available on the effectiveness of HB immunization in field settings.
We therefore conducted this study in two retrospective cohorts of
children, one consisting of those who had received hepatitis B
immunization under field conditions and the other of those who had not.
Methods
The study was conducted during 2010-11 in five
districts of Andhra Pradesh (Rangareddy, Medak, Nizamabad, Karimnagar
and Nalgonda) where hepatitis B immunization had been introduced in
2003-04. In each district, two mandals were identified randomly,
and in each of these, 3-7 primary health centers and sub-centers were
selected.
At each center or sub-center, two groups of children
were enrolled: (a) those who had received three doses of
hepatitis B vaccine as part of infant immunization program (mostly born
in 2003 or 2004), and (b) those who had not received such
immunization (mostly born in 2001 or 2002). The immunization status was
verified using either immunization cards available with the parents or
from records maintained at the primary health center/sub-center.
Children whose immunization status could not be verified or documented,
and those with partial immunization were excluded.
From each child, 3 mL of blood was drawn in a plain
vial, and serum was separated, frozen and quickly transported to a
laboratory where it was stored at -80°C till analysis. The laboratory
was not aware of the origin of individual specimens. Serum specimens
were tested for HBsAg, total anti-HBc and anti-HBs using automated
enzyme linked immunoassays (Roche Elecsys 411). The equipment
automatically scored each test result as positive or negative based on
the controls included in each kit, and calculated a titer for anti-HBs.
To verify the test results, all the sera were retested for HBsAg and a
subset were retested for anti-HBc and anti-HBs, using immunoassay kits
from BioMerieux.
The required sample size for detection of difference
in HBsAg prevalence rates in the immunized and unimmunized children was
calculated using EpiInfo software and the following assumptions:
baseline HBsAg rate among 5-year old children of 3%, expected HBV
carrier rate among immunized children of 1%, acceptable alpha error of
0.05, and study power of 90%. The resulting numbers (1125 in each group)
were corrected for cluster sampling using a ‘design effect’ of 2.0.
Thus, it was proposed to study 2250 immunized and unimmunized children
each.
Prevalence data in the two groups were compared using
chi-square test. For anti-HBs titers, the raw data were converted into
categories (<10, 10-100, 101-1000, and >1000) and compared using
extended Mantel-Haenszel chi-square test for linear trend.
The study protocol was approved by Ethics committees
of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow and
National Institute of Nutrition, Hyderabad. Permission was also obtained
from health department of Andhra Pradesh and respective district health
authorities, and both community (village level) and individual consents
(from one of the parents) were obtained.
Results
The study included 5024 children, of whom 2674 (1357
boys) had received hepatitis B immunization and 2350 (1236 boys) had not
received it.
Table I shows the prevalence of HBsAg,
anti-HBs and anti-HBc among immunized and unimmunized children. The
positivity rate for anti-HBc, a marker of exposure to HBV infection (but
not to hepatitis B vaccine), was lower among immunized children than
among unimmunized children (1.05% vs 1.79%; risk ratio = 0.59
[95% confidence interval = 0.36-0.94]; P = 0.026). Among
immunized children, a larger proportion tested positive for anti-HBs
than unimmunized children (52.9% vs. 17.7%; P<0.001; RR = 2.98
[95% CI 2.71-3.28]), and mean anti-HBs antibody titers were higher (P<0.001)
(Fig. 1).
TABLE I Comparison of Prevalence of Serological Markers Related to Hepatitis B in Immunized and Unimmunized Children
|
Serological marker |
Boys |
Girls |
All children |
P value |
|
Immunized |
Unimmunized |
Immunized |
Unimmunized |
Immunized |
Unimmunized |
|
|
(n=1357) |
(n=1236) |
(n=1317) |
(n=1114) |
(n=2674) |
(n=2350) |
|
|
Anti-HBs |
735 (54%) |
233 (19%) |
680 (52%) |
184 (17%) |
1415 (52.9%) |
417 (17.7%) |
<0.001 |
|
Anti-HBc |
13 (1.0%) |
28 (2.3%) |
15 (1.1%) |
14 (1.3%) |
28 (1.05%) |
42 (1.79%) |
0.026 |
|
HBsAg |
3 (0.2%) |
4 (0.3%) |
1 (0.1%) |
0 (0%) |
4 (0.15%) |
4 (0.17%) |
0.855 |
|
P values relate to comparison of total immunized versus
total unimmunized children. |
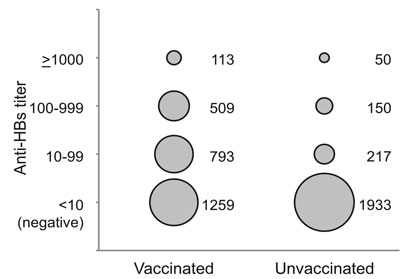 |
|
Fig. 1 Anti-HBs titers (mIU/mL) in
immunized and unimmunized children. The area of each circle is
proportional to the number of children with antibody titer in
each range.
|
In view of dissimilar age distributions of the
immunized and unimmunized children, prevalence rates of anti-HBs and
anti-HBc were compared in various age strata (Table II).
Anti-HBs positivity rates in the immunized cohort showed a progressive
decline (P<0.001) with increasing age. In each age stratum, the
prevalence of anti-HBs was higher among immunized children than in the
unimmunized children; the difference was more marked at younger ages.
The anti-HBc prevalence rates did not show significant differences
between the immunized and unimmunized children in various age strata.
Fig. 2 shows the relationship of anti-HBs titers with age in
the two cohorts. Among immunized children, the proportion of children
with detectable antibody and those with higher titers declined with
increasing age, indicating an age-related antibody decline.
TABLE II Age-wise Frequency of Serological Markers for Hepatitis B among Immunized and Unimmunized Children
|
Age (years) |
Anti-HBs |
Anti-HBc |
|
Immunized |
Unimmunized |
Immunized |
Unimmunized |
|
4 |
0/1 |
— |
0/1 |
— |
|
5 |
295/462 (64%) |
0/1 |
3/462 (0.6%) |
0/1 |
|
6 |
676/1144 (59%) |
3/9 (33%) |
11/1144(1.0%) |
0/9 |
|
7 |
360/768 (47%) |
58/208 (28%) |
10/768 (1.3%) |
4/208 (1.9%) |
|
8 |
42/119 (35%) |
106/619 (17%) |
1/119 (0.8%) |
13/619 (2.1%) |
|
9 |
22/88 (25%) |
94/643 (15%) |
0/88 |
6/643 (0.9%) |
|
10 |
18/76 (24%) |
125/763 (16%) |
3/76 (3.9%) |
15/763 (2.0%) |
|
11 |
2/16 (13%) |
31/107 (29%) |
0/16 |
4/107 (3.7%) |
|
Total |
1415/2674 (53%) |
417/2350 (18%) |
28/2674 (1.0%) |
42/2350 (1.8%) |
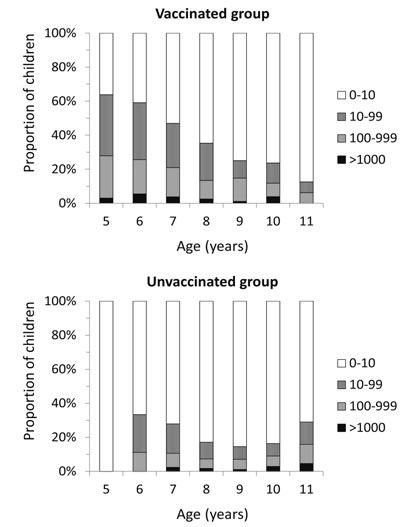 |
|
Fig. 2 Relationship of antibody titers
(mIU/mL) with age among immunized (a) and unimmunized (b)
children.
|
Anti-HBs prevalence rates were similar among boys and
girls (Table I), as were the anti-HBs titers (data not
shown). The difference in anti-HBs positivity rate between immunized and
unimmunized children was observed in each of the five districts (data
not shown).
Retesting of 700 sera for anti-HBc and anti-HBs
using kits from BioMerieux revealed results identical to those for
the original testing. Retesting of all the 5024 sera for HBsAg using the
BioMerieux kits showed positive test results for 14 and borderline
results for 10 specimens; the positive specimens included all the 8
specimens that had tested positive for HBsAg using the Roche kits. Of
the 8 children who tested positive in both the assays, 7 were positive
for anti-HBc. However, of the remaining 16 children who were positive (n=6)
or borderline (n=10) on BioMerieux assay, only one was positive
for anti-HBc.
Discussion
In this retrospective cohort study, children who had
received HB immunization as part of the universal immunization program
in a field setting in Andhra Pradesh, India, showed a higher rate of
anti-HBs positivity, and a reduced rate of anti-HBc positivity, than a
cohort of children from the same area who had not received such
immunization.
Presence of anti-HBs antibodies, which protect
against HBV infection, can be related either to hepatitis B vaccination
or to prior HBV infection. Their higher prevalence in the immunized
cohort, despite a lower anti-HBc seroprevalence in this group, indicates
that the higher rate of anti-HBs in the immunized cohort was the effect
of hepatitis B immunization. This implies that administration of
hepatitis B vaccine in the Indian field setting did lead to induction of
a protective antibody response against HBV. A significantly lower anti-HBc
positivity rate in the immunized cohort in our study indicates that
hepatitis B immunization in the Indian field setting was effective in
reducing HBV transmission, thus justifying the decision to include this
vaccine in the National immunization program.
Reduction in the anti-HBs seropositivity rate in the
immunized cohort with increasing age is related to progressive decline
in the titer of this antibody with time with its eventual disappearance
in a subset of persons who have received hepatitis B vaccine. Follow-up
studies in several countries have shown that at 5-7 years after the
completion of a 3-dose immunization schedule, only 60% to 85% of
vaccinees had anti-HBs titer above the commonly-used cut-off of 10 mIU/mL
[10-14]. Most of these studies were done in populations with high
background rate of transmission of HBV. In India, an area with
intermediate HBV endemicity, such decline of anti-HBs response is
expected to be more pronounced. However, despite the disappearance of
anti-HBs, such immunized individuals continue to be protected against
clinical illness as well as chronic HBV infection on exposure to this
virus [15-17]. Thus, the proportion of children in the immunized cohort
who are protected against HBV infection would be expected to be higher
than the observed prevalence of anti-HBs antibody in this group.
Our study has some limitations. First, we failed to
find a reduction in seroprevalence of HBsAg, a marker of chronic HBV
infection, in the unimmunized cohort compared to the unimmunized cohort.
There was a very low HBsAg seroprevalence in our cohort, as compared to
earlier data from Andhra Pradesh [10]. This, along with a low anti-HBc
positivity rate indicates that HBV transmission rate in the study
population was much lower than our assumption, precluding detection of
any difference in HBsAg positivity rates a that may have existed. This
low transmission rate in our study area could be due to selection of a
rural population for our study (whereas previous HBV seroprevalence
studies are mainly from urban areas) [7], selection of villages with
good immunization records (which may have had better health and medical
care practices, and hence lower HBV transmission); and recent emphasis
on safe injection practices in Andhra Pradesh. The anti-HBs positivity
in our unimmunized cohort was also higher than expected. This was
unlikely to be due to exposure to hepatitis B vaccine since anti-HBc
rate in these children was low, and indicates a high rate of hepatitis B
immunization outside the universal childhood immunization program in the
unimmunized cohort.
It is important to use evidence to monitor and guide
national immunization programs. Data from our study would be useful in
this direction. Despite some limitations, our data indicate that
hepatitis B immunization in field setting was effective in inducing
protection against HBV infection and in reducing its transmission.
Though we were unable to show a significant reduction in hepatitis B
carrier rate, it would be useful to undertake similar studies in other
parts of India where transmission of HBV is more frequent.
In conclusion, our study shows that hepatitis B
immunization as part of Universal infant immunization program in the
Indian population is effective in reducing the rate of HBV infection.
These data should be useful to policy makers and health administrators
to guide the national immunization program.
Acknowledgement: Director, National Institute of
Nutrition, Hyderabad.
Contributors: RA: study design, data acquisition,
data analysis, writing the first draft and revising it critically; JJB:
data acquisition, critical revision of the manuscript; RH: study design,
data acquisition, critical revision of the manuscript; AVR and DS: data
acquisition, critical revision of the manuscript; TK: data acquisition,
drafting the manuscript, critical revision of the manuscript. All
authors approved the final version.
Funding: Indian Council of Medical Research, New
Delhi. Competing interests: None stated.
|
What is Already Known?
• Universal hepatitis B immunization is
useful in preventing HBV infection in populations with high
endemicity for hepatitis B virus infection.
What This Study Adds?
• Inclusion of hepatitis B vaccine in
Universal childhood immunization program of India has led to an
increase in immunity against hepatitis B and protection against
hepatitis B virus infection.
|
References
1. McMahon BJ. Epidemiology and natural history of
hepatitis B. Semin Liver Dis. 2005;25:3-8.
2. McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender
TR, Francis DP, et al. Acute hepatitis B virus infection:
Relation of age to the clinical expression of disease and subsequent
development of the carrier state. J Infect Dis. 1985;151:599-603.
3. World Health Assembly. Resolution WHA 45.17.
Immunization and Vaccine Quality. Geneva: World Health Assembly, 1992.
4. World Health Organization. Hepatitis B vaccines:
WHO position paper. Wkly Epidemiol Rec. 2009;84:405-19.
5. Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS,
et al. Universal hepatitis B vaccination in Taiwan and the
incidence of hepatocellular carcinoma in children. Taiwan Childhood
Hepatoma Study Group. N Engl J Med. 1997;336:1855-9.
6. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et
al. Epidemiological serosurvey of Hepatitis B in China – Declining
HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-7.
7. Batham A, Narula D, Toteja T, Sreenivas V, Puliyel
JM. Sytematic review and meta-analysis of prevalence of hepatitis B in
India. Indian Pediatr. 2007;44:663-74.
8. Lahariya C, Subramanya BP, Sosler S. An assessment
of hepatitis B introduction in India: Lessons for roll out and scale up
of new vaccines in immunization programs. Indian J Public Hlth.
2013;57:8-14.
9. World Health Organization. Documenting the Impact
of Hepatitis B Immunization: Best Practices for Conducting a Serosurvey.
Department of Immunization, Vaccines and Biologicals, World Health
Organization, Geneva. 2011. WHO document WHO/IVB/11.08. Available from:
www.who.int/ immunization/documents/who_ivb_11.08/en/ý. Accessed
April 23, 2014.
10. Wainwright RB, Bulkow LR, Parkinson AJ, Zanis C,
McMahon BJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo
population. J Infect Dis. 1997;175:674-7.
11. Lai CL, Wong CY, Yeoh EK, Lim WL, Chang WK, Lin
HJ. Five-year follow-up of a prospective randomized trial of hepatitis B
recombinant DNA yeast vaccine vs. plasma derived vaccine in children:
immunogenicity and anamnestic responses. Hepatology. 1993;18:763-7.
12. Lee PI, Lee CY, Huang LM, Chang MH. Long-term
efficacy of recombinant hepatitis B vaccine and risk of natural
infection in infants born to mothers with hepatitis B e antigen. J
Pediatr. 1995;126:716-21.
13. Stevens CE, Toy PT, Taylor PE, Lee T, Yip HY.
Prospects for control of hepatitis B virus infection: Implications of
childhood vaccination and long-term protection. Pediatrics.
1992;90:170-3.
14. Da Villa G, Pelficcia MG, Peluso F, Ricciardi E,
Sepe A. Anti-HBs responses in children vaccinated with different
schedules of either plasma-derived or HBV DNA recombinant vaccine. Res
Virol. 1997;148:109-14.
15. Banatvala J, Van Damme P, Oehen S. Lifelong
protection against hepatitis B: the role of vaccine immunogenicity in
immune memory. Vaccine. 2001;19:877-85.
16. European Consensus Group on Hepatitis B Immunity.
Are booster immunisations needed for lifelong hepatitis B immunity?
Lancet. 2000; 355:561-5.
17. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC.
What level of hepatitis B antibody is protective? J Infect Dis.
1999;179:489-92.
18. Chandra M, Khaja MN, Farees N, Poduri CD, Hussain
MM, Aejaz Habeeb M, et al. Prevalence, risk factors and genotype
distribution of HCV and HBV infection in the tribal population: A
community based study in south India. Trop Gastroenterol. 2003;24:193-5.
19. Patel DA, Gupta PA, Kinariwala DM, Shah HS,
Trivedi GR, Vegad MM. An investigation of an outbreak of viral hepatitis
B in Modasa town, Gujarat, India. J Glob Infect Dis. 2012;4:55-9.
|
|
|
 |
|

