Neonatal chylothorax is an abnormal accumulation
of lymphatic fluid in the pleural space which can be either
congenital or acquired. Nearly 90% of all in utero pleural
effusions are chylothorax [1]. The estimated incidence of
congenital chylothorax is 4 per lakh [2], with mortality
ranging from 30-50%. [3]. We herein report a late preterm
girl identified antenatally at 31 weeks of gestation with
severe bilateral pleural effusion for which thoracoamniotic
shunt was placed and subsequently diagnosed with congenital
chylothorax after delivery.
A 37-year-old lady,
G3P1A1L1 was admitted at 365/7 weeks for delivery of
hydropic fetus. Antenatal follow up had been uneventful till
31 weeks when ultrasonography showed hydropic changes in the
fetus with bilateral pleural effusion and subcutaneous
edema. A therapeutic fetal pleurocentesis was done with
amniocentesis. Chromosomal analysis and microarray on
amniotic fluid was negative. Mother had a negative indirect
coomb’s test, with serology negative for VDRL, and TORCH.
Parvo Virus PCR was negative, and she had a normal HbA1C and
Hemoglobin electrophoresis. Pleural fluid examination showed
205 leucocytes per cu.mm, 80% lymphocytes, LDH 87 U/L and a
protein of 1.8g/dL. Follow up scan at 33 weeks showed a
return of significant pleural effusion. Rather than opting
for preterm delivery, a right Rodeck thoracoamniotic shunt
was placed. Subsequent USG showed resolution of effusion on
the Right side with lung expansion and satisfactory interval
growth with normal fetal Dopplers. The left pleural effusion
was drained just prior to the delivery.
A female baby
with birth weight of 3015 g was delivered at 365/7 week
gestation by elective LSCS. Baby had signs of labored
breathing at birth, and was intubated and ventilated. Chest
X-ray showed right side pneumothorax and left side pleural
effusion for which bilateral intercostal tube drains (ICD)
were inserted. Pleural fluid was clear exudate (Protein –
2.6 g/dL) with 3638 cells/mm3, predominantly lymphocytes and
1.9% neutrophils. Post ICD insertion baby improved and was
extubated to high flow nasal cannula (HFNC).
Echocardiography and ultrasound abdomen and cranium were
normal. Once feeds were started pleural fluid became milky
in nature. Pleural fluid sent for analysis showed rise in
triglyceride level from baseline 35.8 mg/dL on Day 1 to 134
mg/dL on day 6 confirming the diagnosis of chylothorax.
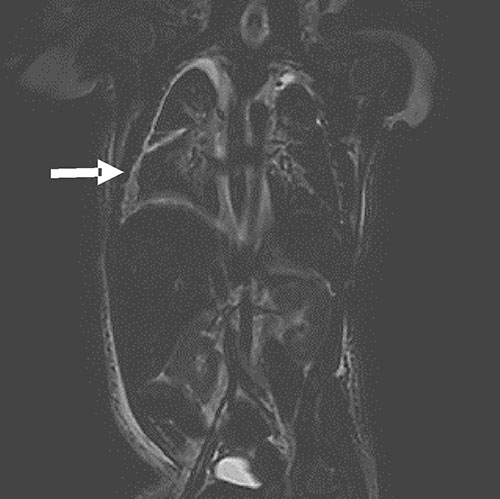 |
| Fig. 1 MRI
image (T2 SPAIR, coronal diffusion weighted
sequence) showing bilateral pleural effusion (R>L),
prominent tortuous lymphatic channels in thoracic
region extending into upper abdomen, with no
evidence of any mediastinal mass. |
MRI Chest was done on Day 8 of life for central
lymphatic anatomy and intrathoracic mass lesions. It showed
prominent tortuous lymphatic channels along with prominent
azygous vein
(Fig. 1). Baby was started on
medium chain triglyceride formula on day 9 of life in view
of chylothorax. Feeds had to be discontinued and parenteral
nutrition restarted along with injection Octreotide on day
12 because of increasing chyle drainage. Following this,
chyle formation reduced and the intercostal drains were
removed on day 16. Post drain removal there was an increase
in pleural effusion (right >left) which was organizing and
non-tappable. Octreotide infusion was increased in view of
persistent collection (at 10 mcg/kg/hr). Immunoglobulin
levels were low for which single dose IVIG was given. On Day
25, lymphoscintigraphy was done to rule out lymphatic
dysplasia, which was reported as normal. Feeds were
restarted on day 28, after which there was worsening in
respiratory distress but there was no increase in pleural
effusion; as monitored by ultrasound. Keeping the
possibility of leaky pulmonary lymphatics causing increase
in pulmonary interstitial fluid, diuretics were added, to
which baby responded well and feeds were gradually
increased. Diuretics were continued till day 41 of life.
Octreotide infusion was tapered gradually. Clinical exome
testing done which showed no pathogenic variants causative
of the phenotype, but variants of uncertain significance
were detected (lymphatic malformation-3, OMIM#613480). Baby
was discharged on day 44 of life on MCT- based formula
(Pregestimil).
Congenital chylothorax can be an
isolated finding or may be associated with genetic
conditions. Early antenatal detection and management by
placement of fetal pleuroamniotic shunt improves perinatal
outcome by avoiding complications due to pulmonary
hypoplasia [4]. Irrespective of the cause, initial postnatal
management consists of drainage of pleural fluid,
appropriate ventilation, total parenteral nutrition and
dietary modi-fication (conservative approach). Medication
and surgery may be required in refractory cases. Most
chylothorax cases improve spontaneously because of the
natural course of the disease. In newborns it is important
to distinguish neonatal chylothorax from congenital
lymphatic dysplasia, as the latter is difficult to treat and
has poorer prognosis.
As increased chyle formation
is associated with immunological and nutritional
complications, we had started octreotide on day 12. On
reviewing literature [5,6] we could not find any practice
recommendations for use of octreotide. The lymphatic
malformation-3 may explain the localized edema in neck,
genitals and probably in lungs, which persisted at time of
tapering of octreotide infusion.
To conclude,
optimum management of such cases is still a matter of
debate, but prenatal evaluation and management is associated
with improved survival. Postnatally we should follow
conservative approach for few weeks to give enough time for
the lymphatics to heal and develop collaterals [6].
Refractory cases would require additional therapy.
Contributors: RS: drafted the case report and revised it
critically for important intellectual content, involved with
management of case; TJA: revision of case report for
important intellectual content, involved with case
management, approved the version to be published; SG:
involved with case management, approved the version to be
published.
Funding: None; Competing interest: None
stated.
REFERENCES
1. Rocha
G, Fernandes P, Rocha P, Quintas C, Martins T, Proença E .
Pleural effusions in neonate. Acta Paediatr. 2006;95:791-8.
2. Bialkowski A, Poets CF, Franz AR. Congenital
chylothorax: A prospective nationwide epidemiological study
in Germany. Arch Dis Child Fetal Neonatal Ed. 2015;100:F169.
3. Dorsi M, Giuseppi A, Lesage F, Stirnemann J, De Saint
Blanquat L, Nicloux M, et al. Prenatal factors associated
with neonatal survival of infants with congenital
chylothorax. J Perinatol. 2017;38:31-4.
4. Lee C,
Tsao P, Chen C, Hsieh W, Liou J, Chou H. Prenatal therapy
improves the survival of premature infants with congenital
chylothorax. Pediatr Neonatol. 2016;57:127-32.
5.
Bellini C, Cabano R, De Angelis L, Bellini T, Calevo M,
Gandullia P, et al. Octreotide for congenital and acquired
chylothorax in newborns: A systematic review. J Paediatr and
Child Hlth. 2018;54:840-7.
6. Beghetti M, La Scala G,
Belli D, Bugmann P, Kalangos A, Le Coultre C. Etiology and
management of pediatric chylothorax. J Pediatr.
2000;136:653-8.

