In children and adolescents, renal artery
stenosis (RAS) accounts for up to 10% of the secondary causes of
hypertension. Glomerular disease and renal parenchymal scarring
are responsible for an additional sixty percent [1-3]. RAS is a
heterogeneous disease process that includes intrinsic lesions of
the renal arteries, extrinsic compressive masses, and
intraluminal thrombosis that impede renal blood flow [4].There
is an increased risk of developing cardiac and neurologic
complications in adulthood (i.e. myocardial infarction,
stroke) when childhood onset renovascular hypertension (RVHTN)
is not adequately managed [5]. There needs to be a high index of
clinical suspicion to appropriately diagnose and manage RVHTN in
children. Unlike adults where 70-80% of patients have largely
non-correctable atherosclerotic lesions, children with RAS often
have lesions that are amenable to therapeutic intervention [1].
The protean clinical and laboratory manifestations of RVHTN in
children creates a significant challenge in diagnosis that may
contribute to chronic kidney disease and target organ damage
[5]. Given these difficulties, there is a need for a
standardized approach to the diagnosis and management of RVHTN
in children and adolescents [6]. In this review, we will examine
the clinical findings, diagnostic studies, management, and
intervention for pediatric RAS-associated hypertension. This
information will hopefully contribute to future standardized
recommendations to the approach and management of RVHTN in
children and adolescents.
ETIOLOGY
In contrast to adults where the main cause of RAS is from
atherosclerosis, the etiologies in the pediatric population vary
by disease process and by geography. The major contributor to
pediatric RAS in North America and Europe is fibromuscular
dysplasia (FMD), as opposed to Takayasu arteritis (TA) in Asia
and South Africa [7,8]. The etiologies of RAS in children and
adolescents are all summarized in Box I [7,9].
Box I Causes of
Renal Artery Stenosis in Children and Adolescents
|
|
Non-inflammatory
Fibromuscular dysplasia
Mid-aortic syndrome
Inflammatory
Takayasu arteritis
Kawasaki disease
Polyarteritis nodosa
Syndromes
Neurofibromatosis type 1
Tuberous Sclerosis
Williams’ syndrome
Marfan’s syndrome
Alagille syndrome
Turner syndrome
Congenital rubella
Localized tissue damage
Trauma
Radiation
Extra-luminal
Compression by mass
Wilms’ tumor, Neuroblastoma, Other
Intra-luminal
Catheter-related thromboembolic disease
Hypercoagulable states – nephrotic syndrome
Surgical
Transplant renal artery stenosis
Idiopathic
|
CLINICAL CLUES
RAS is often a ‘silent’ diagnosis with many non-specific
symptoms. We aim to summarize the most recent findings
acknowledging the paucity of clinical features while
understanding the concern for complications from long-standing
renovascular-associated HTN.
History:
The age of the child can be crucial in directing the
differential of pediatric RAS. As an infant, there is a higher
pre-test probability of having a thrombosis or emboli from a
catheter site as opposed to the young child where syndromes and
inflammation play a larger role [10]. The odds of detecting a
secondary cause of hypertension are inversely proportional to
the age of the child, creating an emphasis on early diagnosis
[11]. In FMD, the mean age of diagnosis was 8.4 years with a
range from 16 days to 17 years [12]. Most children often report
non-specific symptoms including headache, and abdominal, and
flank pain [12]. In contrast to adults, children may find it
difficult to characterize common symptoms associated with
hypertension, such as tinnitus or blurry vision [12]. A
retrospective study in Israel noted behavioral changes within
the 3-12 months prior to diagnosis of RVHTN that included
hyperactivity, restlessness, and attention deficits [13]. This
creates a conundrum for physicians that are evaluating these
patients, as increased blood pressure can be missed or
incorrectly diagnosed.
Family history and genetics: When referring to the etiologies of RAS, one of
the largest categories include RAS-associated syndromes (Web
Table I). Although the discovery of new genes
continue to grow, data has shown that approximately 11%-60% of
RAS cases are familial [7]. In a cohort of 93 children with RAS
and mid-aortic syndrome (MAS) in Canada, 26% had an underlying
genetic disease, 24% had an inflammatory process, and 50% were
idiopathic [10]. Of the children with genetic conditions, about
40% had neurofibromatosis type 1 (NF-1) and the remaining had
William syndrome or Alagille syndrome [10]. Within the FMD
registry, there are a significant number of pediatric patients
with a family history of FMD in comparison to the adults,
supporting a stronger familial genetic inheritance in pediatric
FMD-related vascular disease [12,14]. In addition, children and
adolescents with underlying genetic or inflammatory syndromes
are more likely to have extrarenal vascular involvement
including visceral and proximal aortic branches [10].
Blood pressure measurements:
The physical examination in children and
adolescents with RAS is most often unrevealing, which can cause
a delay in diagnosis. The most common finding is of isolated
hypertension. It is estimated that 26-70% of renovascular
disease presents with hypertension in an otherwise asymptomatic
child [15,16]. A report from the Midwest pediatric nephrology
consortium in 2010 found no difference in age, weight
distribution, or stage of hypertension when trying to
differentiate between primary and secondary hypertension [17].
However, children with RAS typically present with stage 2
hypertension [18]. The likelihood of identifying a secondary
cause of hypertension such as RAS has been found to be directly
related to the degree of blood pressure elevation [11,19].
Other factors that must be taken into consideration
include when and how the blood pressure measurements are taken
in the clinical setting. Children in the United States start
getting blood pressure measurements at the age of three unless
they fall into a high-risk category. Unfortunately, some
children may be referred with a history of elevated blood
pressures after several clinic visits without intervention or
evaluation due to the concern of inaccurate readings in an
asymptomatic child [7]. Appropriate blood pressure readings are
essential, which include the following: (i) appropriate
cuff size; (ii) sitting position; (iii) right
upper extremity; (iv) calm environment; and (v)
after 3-5 minutes of rest. When the blood pressure is found to
be elevated for the first time, four extremity blood pressures
are obtained to evaluate for coarctation of the aorta and MAS
[9].
Physical examination:
Physical findings of RAS-associated syndromes are detailed in
Web Table
I. Children with Takayasu arteritis typically have
consti-tutional symptoms and signs secondary to inflammation.
This includes arthralgia, skin rashes, abdominal bruits, and
absence of pulses [20]. In FMD, bruits can sometimes be heard
overlying the epigastrium (7.4%), carotid arteries (7.4%), and
flank (7.7%) [12]. For patients with MAS, a mid-abdominal murmur
is a classic finding [4].
There is a subset of pediatric patients that
present with secondary signs of target organ damage related to
hypertension, including neurological (10-15%) and cardiac
findings (7%) [4,15]. The neurological symptoms can range from
headache, seizures, stroke, to cranial nerve palsies [7,21].
Bell palsy is the most commonly identified cranial nerve palsy
[15]. One study showed that older children are more likely to
have cardiac findings of palpitations, murmur, or signs of
congestive heart failure, with 10% of them having an underlying
syndrome [3,12]. Ocular findings are specific to syndromes such
as Alagille, but can be present as a non-specific sign of
hypertensive retinopathy [3].
LABORATORY EVALUATION
To evaluate for RVHTN, laboratory and imaging diagnostic tests
need to be ordered in a step-wise fashion. An initial basic
metabolic panel is appropriate to determine if there are signs
of renal dysfunction (azotemia, elevated creatinine) or
electrolyte derangements defined by hyponatremia, hypokalemia,
and alkalosis suggestive of RAS.
Sodium:
There have been a few pediatric cases of unilateral renal artery
stenosis that presented with marked hyponatremia. This is termed
hypertensive hyponatremic syndrome (HHS) [22]. The hyponatremia
is postulated to occur from hyperactivation of the
renin-angiotensin-aldosterone system (RAAS) with substantial
increase in angiotensin II production directly causing arterial
vasoconstriction. This results in a pressure natriuresis from
the contralateral kidney that has normal function. The severity
of the hyponatremia can be compounded by a reactive secretion of
anti-diuretic hormone from the transient volume depletion
[22,23].
Potassium:
The presence of hypokalemia is rare, but is seen in the setting
of unilateral RAS. With decreased perfusion to the affected
kidney there is activation of the RAAS system with secondary
hyperaldosteronism resulting in hypokalemia due to excessive
urinary potassium loss [24]. Ultimately, this can be corrected
with either improvement of the renal ischemic state or with
blockade of the RAAS.
Creatinine:
In unilateral disease, the serum creatinine concentration
remains normal through compensation of the healthy kidney.
However, monitoring is essential. Bilateral disease can have
decreased renal function in the setting of hypoperfusion and can
be exacerbated if angiotensin-converting enzyme inhibitors
(ACEi) and angiotensin-receptor blockers (ARBs) are initiated
[24]. After anti-hypertensive medication is started for BP
control in children with RAS, a metabolic panel including
creatinine should be checked within 1-2 weeks to ensure that
there is no evolving kidney injury.
Urinalysis:
With unilateral RAS and prolonged ischemia to a single kidney,
there may be compensatory hypertrophy of the contralateral
kidney, resulting in glomerular hyperfiltration. This phenomenon
combined with chronic activation of the RAAS can lead to
proteinuria and glycosuria, biomarkers of sub-clinical damage to
an otherwise normal kidney.
Plasma renin activity (PRA): The PRA level is dependent on age, sodium intake,
posture, and oscillates in a diurnal pattern. All these factors
make a PRA value difficult to interpret. It can also be
suppressed in primary essential hypertension in African
Americans and various forms of monogenetic hypertension (e.g.,
Liddle syndrome). Studies have shown normal PRA values in
20%-37% of patients with unilateral RAS [25]. With bilateral
RAS, the child is likely to have normal renin and aldosterone
levels [1]. This is due to volume-dependent hypertension, after
initial RAAS activation and volume retention there is subsequent
suppression of renin release [26,27]. Given the low predictive
value of PRA, further investigations need to be performed if
there is a high index of clinical suspicion for RAS [27].
RADIOLOGICAL IMAGING
There is no single screening, radiological study that can
effectively exclude all the causes of RAS in children. There is
an ongoing evaluation to identify modalities that are more
sensitive and specific in diagnosing RAS (Table I)
[7,8,28,29]. This is important from the patient perspective
given that the gold standard for the diagnosis of RAS in
children and adolescents continues to be the percutaneous
angiogram, which is an invasive procedure.
Table I Imaging Modalities for Renal Artery Stenosis
|
Modalities of imaging |
Advantages |
Disadvantages |
Sensitivity |
Specificiy |
|
Renal Bladder Ultrasound (RBUS) with Doppler |
Easy availability, non-invasive,fast, no radiation, simple, lowcost |
Operator-dependent, age- dependent cooperation, body habitus, may miss small lesions high false positive and false negative |
27-63% |
70-100% |
|
Magnetic resonance angiography (MRA) |
No radiation, improved imagequality |
Limited intrarenal vessel visualization, longer study, may require anesthesia, compro-mised by respiration |
62-98% |
70-96% |
|
CT angiography (CTA) |
Fast, improved image quality, not compromised by respiration |
Requires radiation, limited intrarenal vessel visualization |
64-100% |
62-97% |
|
Renal scintigraphy |
Non-invasive, inexpensive |
Low predictive probability,reduced accuracy in renal failure, does not visualize the vessels; inconsistent data |
59-73% |
68-88% |
|
Digital subtraction angiography (DSA) |
Detailed imaging of aorta and all branches, can transiation to a therapeutic intervention |
Radiation, requires anesthesia |
100% |
100% |
Renal bladder ultrasound (RBUS) with doppler: A RBUS is the appropriate first line of imaging
given its advantages (Table I) and the ability to
assess for other secondary causes of hypertension including a
mass, venous thromboembolism, renal dysplasia, and scarring
[30]. It can provide valuable assistance in monitoring
progression of RAS after angioplasty by specifically measuring
the peak systolic velocity (PSV) and resistive indices of the
affected vessel [31]. The many limitations of the doppler US
include the difficulty in assessing small vessels, age-dependent
cooperation, body habitus and operator proficiency. In children,
when compared to angiography, it has a 27% sensitivity as a
diagnostic alternative. Although, there are reports of better
specificity ranging from 70-100% in both adult and pediatric
populations [12,14]. Contrast enhanced ultrasound, a relatively
newer modality, has shown improved sensitivity ranging from
79-100% for diagnosis of RAS and may be a better initial
screening study [32].
Magnetic resonance angiography (MRA): An MRA provides detailed renal size and blood
flow without exposure to radiation [14]. This is an appropriate
study to assess the aorta and main renal arteries with limited
visualization of intrarenal vessels. In adult studies, MRA’s has
shown to have a sensitivity of 92-98% and specificity of 70-96%
in diagnosis of renovascular disease, particularly for
atherosclerotic-associated RAS [33]. Limitations of MRA include
its inability to assess involvement of segmental renal vessels.
It can exaggerate the degree of narrowing within the main renal
artery given lack of adequate spatial resolution compared with a
computed tomography angiography [34]. In a pediatric cohort
comparing US, MRA, and CTA in 25 patients with FMD, the MRA
imaging study demonstrated a sensitivity of 62.5% for RAS
detection with 100% specificity [8].
Computed tomography angiography (CTA): A CTA exposes the patient to radiation; however,
radiation minimization protocols can be used to reduce this
unwanted effect. CTA can depict the renal arteries with its
first branches, kidney size, parenchymal wall thinning/scarring,
and is not compromised by respiration as opposed to an MRA [29].
The CTA has proven to be the best and fastest alternative to an
angiography in detecting RAS and renal artery aneurysms. The
sensitivity has been shown to be as high as 84.2% in a pediatric
study [8]. It can specifically detect thin webs that can be
present in FMD that may be missed on MRA [29]. Within the adult
population, it rivals an MRA with a sensitivity range of 64-100%
and specificity range of 62-97% [33]. Recent studies show that
reconstruction techniques of CTA can reduce noise and improve
accuracy of vessel diameter measurements [35,36].
Renal scintigraphy:
Renal scintigraphy is a nuclear medicine study that is
non-invasive and safe. A radioactive tracer,
99m-technetium-dimercaptosuccinic acid (99m
Tc-DMSA) or 99m-Tc-mercaptoacetyl-trigly-cine (99mTc-MAG3), is used to assess renal
function with administration of an angiotensin-converting-enzyme
inhibitor (ACEi). The renogram curve can suggest vessel
narrowing by demonstrating time to peak activity and delayed
washout. It has a low predictive probability and is an image
that does not directly visualize the vessels. The results have
continued to be inconsistent and the test has fallen out of
favor in comparison to the prior modalities [29].
Renal vein renin sampling:
Renal vein renin sampling is an invasive test that entails
taking a blood sample from the inferior vena cava and comparing
it to samples taken from the main renal veins. This test
requires an anesthesiologist, and can be performed in
conjunction with a diagnostic angiography via a femoral
approach. The data allows one to identify the ischemic focus,
which can be localized to the specific kidney that is involved.
Given that imaging has progressed over the years and that
selective renal vein sampling has low sensitivity (74%) and
specificity (59%), it is not as commonly used [37]. In adults,
the American college of cardiology/American heart association
guidelines no longer recommend using it for detection of RAS
[38].
Digital subtraction angiography (DSA): Renal angiography continues to be the gold
standard and provides detailed imaging of the aorta and all of
its branches. This entails injection of contrast via a
percutaneous catheter into the aorta and main renal arteries. It
is the most invasive out of all the tests, requires radiation
exposure, and anesthesia for children and adolescents. The
benefit of the angiogram includes the detailed vasculature that
highlights occlusion of renal vessels and collateral vessels. It
can be transitioned to a therapeutic intervention (angioplasty)
or used to provide exact information for next steps in the
management of RAS. A retrospective study was performed to
evaluate the accuracy of US, MRA, and CTA in comparison to a DSA
in 127 children with suspected RAS. The study demonstrated low
sensitivities for the former modalities: 63%, 88%, and 80%,
respectively [33]. Thus, the DSA remains the cornerstone for
accurate diagnosis or exclusion of RAS.
MANAGEMENT OF RAS
Initial Blood Pressure Management
Pre-intervention is directed at blood pressure management with
an appropriate antihypertensive agent and controlled reduction.
Until bilateral RAS or unilateral RAS to a single kidney is
excluded, treatment should be initiated with a vasodilator
and/or a beta blocker. Once the former is excluded, an ACEi or
ARB can be started. RAAS blockers are relatively contraindicated
in critical main RAS and bilateral RAS, but can be used with
segmental stenotic lesions [18]. In addition to in-office blood
pressure monitoring, 24-hour ambulatory blood pressure
monitoring (ABPM) can provide valuable information about
control. In a study of 10 children with RAS on antihypertensive
treatment with normal in-clinic blood pressure readings only two
had adequate control by 24-hour ABPM [39]. Fig. 1
outlines the initial evaluation and management of children with
suspected RAS.
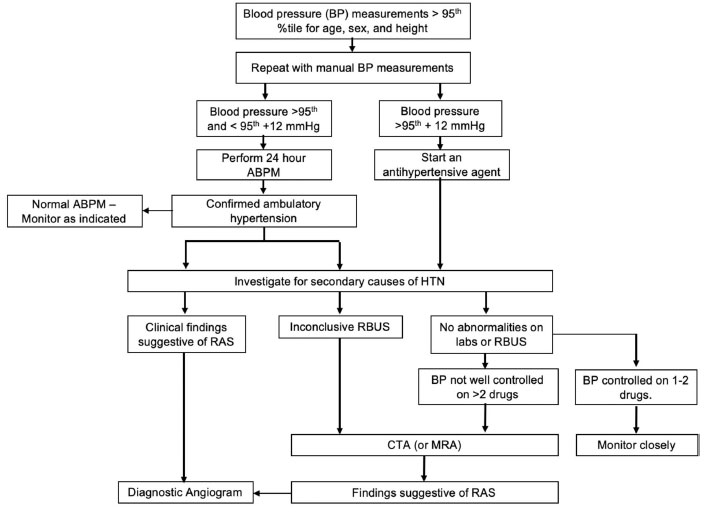
ABPM: Ambulatory blood pressure monitoring, RBUS: Renal
bladder ultrasound; RAS: Renal artery stenosis, MRA:
Magnetic resonance angiography, CTA: CT angiography,
HTN: Hypertension. |
| Fig. 1 An approach
to the diagnosis of renal artery stenosis in children
and adolescents. |
Treatment Options
Treatment of RAS includes continuation of medical therapy with
no intervention, or intervention through percutaneous
transluminal angioplasty (PTA) or surgery. The goal of invasive
treatment is to preserve renal function with restoration of
renal perfusion, and to aid with blood pressure control [40].
The therapeutic decision algorithm is influenced by the patient
anatomy, disease etiology, and clinical expertise of the
institution [1].
Continuation of Medical Therapy
Continuation of medical
therapy includes patients who are still being evaluated for RAS
and those who are not eligible for angioplasty or surgical
intervention due to unacceptable risk or not technically
feasible. In addition, at least half of the children that
undergo an interventional radiology or surgical procedure will
require continued medical therapy [8]. Patients who are not
deemed eligible for intervention tend to have a poorer response
to initial medical treatment and will require use of multiple
antihypertensive agents from different classes to control their
blood pressure. A trial of ACEi or ARB can be used in these
patients with careful monitoring of renal function and after
discussion about the risks and benefits with the family [7].
Taking a non-invasive approach to the management of blood
pressure presents its own set of challenges related to
medication adherence and drug side effects [1]. In small
children, it may be prudent to wait for the child to complete
puberty prior to attempting an intervention, this is
particularly true for children with mid-aortic syndrome [41].
Interventional Radiology
Many pediatric centers use PTA
as first line therapy for RAS lesions of
£10 mm, but a surgical approach is appropriate when
the RAS is complicated by stenotic lesions >10 mm, multiple
stenosed large vessels, or bilateral RAS [42-44]. PTA is
performed under general anesthesia with femoral or brachial
artery access to introduce a long vascular sheath or a guide
wire to the renal arteries. Intra-procedure anticoagulation is
performed with heparin. The balloon diameter used for dilation
varies with age and vessel size, which can be determined by
measuring the adjacent, normal renal artery distal to the
post-stenotic dilation or contra-lateral artery [44]. In
resistant stenoses, use of the cutting balloon has been most
successful in our center (Fig. 2 and 3).
Renal artery stenting is another option when there are lesions
that show elastic recoil or restenosis after conventional or
cutting balloon angioplasty [44]. However, this is
controversial, given that the long-term outcome is unknown
including in-stent restenosis rates and limitation of future
surgeries. In our institution, renal artery stent placement is
avoided and is only used in emergent situations as a temporary
bridge to surgical repair.
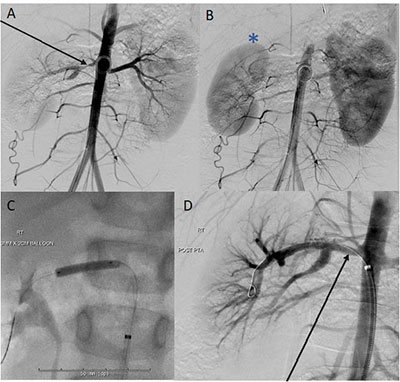 |
| Fig. 2 (a) and (b):
Marked stenosis near the origin of the right main renal
artery (arrow) which supplies the upper and mid kidney
with diminutive size and delayed perfusion of the right
kidney (star) compared to the left; (c) and (d) Panel C
and D: Successful, uncomplicated cutting balloon
angioplasty of a tight right main renal artery stenosis
in a 5-year-old girl with renovascular hypertension.
Perfusion to the right kidney normalized on angiography
following the angioplasty. |
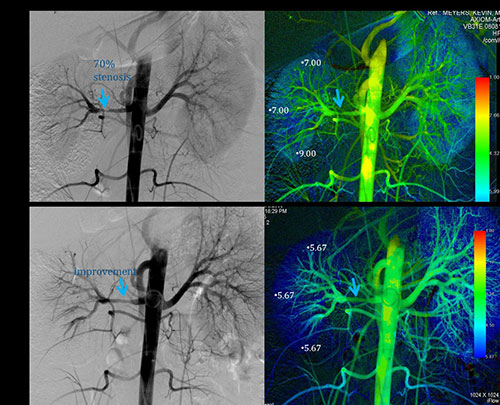 |
| Fig. 3
Sixteen-year-old female with hypertension and main right
renal artery stenosis. Post angioplasty with 4 mm
balloon significant improvement is noted in the >70%
stenosis with improved time to parenchymal perfusion
(TTP) noted on color parametric imaging with the patient
now normotensive and off antihypertensive medications.
|
Adult studies have shown that the benefit from a
primary angioplasty was as high as 93-98%, and in children cure
or improvement is seen in over 50% of cases [3,43].
Complications associated with PTA include arterial spasm,
dissection, and perforation of vessel [7]. Patients who have an
inadequate response to PTA usually develop worsening
hypertension within months post procedure [44].
Surgery
Surgical approaches are primarily used when there is refractory
hypertension after angioplasty, conservative medical therapy, or
vascular lesions that are not amenable to angioplasty [7,44].
Patients with MAS, long segment stenosis, and aneurysms are best
treated with a surgical approach. Surgical procedures include
renal artery re-implantation onto an adjacent portion of normal
aorta and aorto-renal bypass that uses a conduit of autogenous
vessel or prosthetic material to connect the renal artery beyond
the stenosis to the aorta. Patch aortoplasty and aortic bypass
can be used for MAS [45].
In a published series of children and adolescents,
surgical intervention has a cure rate of arterial hypertension
in 70-82% and improved blood pressure measurements in 12-27%
[41,46]. Cure rates in smaller case series are reported between
36-70% [44-46]. In select cases with a poorly or nonfunctional
kidney and unilateral disease, a nephrectomy can be performed
that can result in long-term normotension [47].
Conclusions
RVHTN is an important cause of secondary hypertension in
children and adolescents. A heightened clinical suspicion for
RAS should be present when blood pressure control is refractory
to multiple antihypertensive medications, an abdominal bruit is
present, or in the setting of RAS associated syndromes. Medical
management includes antihypertensive drug therapies for adequate
blood pressue control. Meanwhile, a multi-disciplinary team is
essential in providing individualized care, and guidance on
interventional radiology/surgical procedures.
Contributors:
LV: corresponding author of the paper, coordination of the
review article from planning, literature review, drafted the
manuscript, designed tables, and finalized the submission; AMC:
creation of figures, provided help with literature review and
design of the paper, reviewed the manuscript; and KM: design and
format of the paper, designed tables, reviewed the manuscript,
and provided extensive input in finalized submission. All
authors approved the final version of manuscript.
Funding: None; Competing interest: None stated.
References
1. Lobeck IN, Alhajjat AM,
Dupree P, Racadio JM, Mitsnefes MM, Karns R, et al. The
management of pediatric renovascular hypertension: A single
center experience and review of the literature. J Pediatr Surg.
2018;53:1825-31.
2.
Textor SC, Lerman L. Renovascular hypertension and
ischemic nephropathy. Am J Hypertens. 2010;23:1159-69.
3.
Shroff R, Roebuck DJ, Gordon I, Davies R, Stephens S,
Marks S, et al. Angioplasty for renovascular hypertension
in children: 20-year experience. Pediatrics. 2006;118:268-75.
4.
McTaggart SJ, Gulati S, Walker RG, Powell HR, Jones CL,
Gelati S. Evaluation and long-term outcome of pediatric
renovascular hypertension. Pediatr Nephrol. 2000;14: 1022-9.
5.
Kanitkar M. Renovascular hypertension. Indian Pediatr.
2005;42:47-54.
6.
Lee Y, Lim YS, Lee ST, Cho H. Pediatric renovascular
hypertension: Treatment outcome according to underlying disease.
Pediatr Int. 2018;60:264-9.
7.
Tullus K, Brennan E, Hamilton G, Lord R, McLaren CA,
Marks SD, et al. Renovascular hypertension in children.
Lancet. 2008;371:1453-63.
8.
Louis R, Levy-Erez D, Cahill AM, Meyers KE. Imaging
studies in pediatric fibromuscular dysplasia (FMD): A
single-center experience. Pediatr Nephrol. 2018;33:1593-9.
9.
Gulati S. Childhood hypertension. Indian Pediatr.
2006;43:326-33.
10.
Rumman RK, Matsuda-Abedini M, Langlois V, Radhakrishnan
S, Lorenzo AJ, Amaral J, et al. Management and outcomes
of childhood renal artery stenosis and middle aortic syndrome.
Am J Hypertens. 2018;31:687-95.
11.
Bartosh SM, Aronson AJ. Childhood hypertension. An update
on etiology, diagnosis, and treatment. Pediatr Clin North Am.
1999;46:235-52.
12.
Green R, Gu X, Kline-Rogers E, Froehlich J, Mace P, Gray
B, et al. Differences between the pediatric and adult
presentation of fibromuscular dysplasia: Results from the US
Registry. Pediatr Nephrol. 2016;31:641-50.
13.
Krause I, Cleper R, Kovalski Y, Sinai L, Davidovits M.
Changes in behavior as an early symptom of renovascular
hypertension in children. Pediatr Nephrol. 2009;24:2271-4.
14.
Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray
BH, et al. Fibromuscular Dysplasia: State of the Science
and Critical Unanswered Questions: A Scientific Statement from
the American Heart Association. Circulation. 2014;129:1048-78.
15.
Deal JE, Snell MF, Barratt TM, Dillon MJ. Renovascular
disease in childhood. J Pediatr. 1992;121:378-84.
16.
Estepa R, Gallego N, Orte L, Puras E, Aracil E, Ortuño J.
Renovascular hypertension in children. Scand J Urol Nephrol.
2001;35:388-92.
17.
Kapur G, Ahmed M, Pan C, Mitsnefes M, Chiang M, Mattoo
TK. Secondary hypertension in overweight and stage 1
hypertensive children: A midwest pediatric nephrology consortium
report. J Clin Hypertens (Greenwich). 2010;12:34-9.
18.
Sharma S, Meyers KE, Vidi SR. Secondary forms of
hypertension in children: Overview. In: Flynn J,
Ingelfinger JR, Redwine K, ed. Pediatric hypertension
[Internet]. Cham: Springer International Publishing; 2016. p.
1-20. Available from:
https://doi.org/10.1007/978-3-319-31420-4_21-1. Accessed
October 13, 2019.
19.
NAtional High Blood Pressure Education Program Working
Group on High Blood Pressure in Children and Adolescents. The
Fourth Report on the Diagnosis, Evaluation, and Treatment of
High Blood Pressure in Children and Adolescents. Pediatrics.
2004;114:555-76.
20.
Kothari S. Takayasu’s arteritis in children – a review.
Images Paediatr Cardiol. 2001;3:4-23.
21.
Kirton A, Crone M, Benseler S, Mineyko A, Armstrong D,
Wade A, et al. Fibromuscular dysplasia and childhood
stroke. Brain. 2013;136:1846-56.
22.
Mukherjee D, Sinha R, Akhtar MS, Saha AS. Hyponatremic
hypertensive syndrome - A retrospective cohort study. World J
Nephrol.. 2017;66:41-4.
23.
Atkinson AB, Morton JJ, Brown JJ, Davies DL, Fraser R,
Kelly P, et al. Captopril in clinical hypertension.
Changes in components of renin-angiotensin system and in body
composition in relation to fall in blood pressure with a note on
measurement of angiotensin II during converting enzyme
inhibition. Br Heart J. 1980;44:290-6.
24.
Ruby ST, Burch A, White WB. Unilateral renal artery
stenosis seen initially as severe and symptomatic hypokalemia:
Pathophysiologic assessment and effects of surgical
revascularization. Arch Surg. 1993;128:346-8.
25.
Herrmann SMS, Textor SC. Diagnostic criteria for
renovascular disease: Where are we now? Nephrol Dial Transplant.
2012;27:2657-63.
26.
Krebs C, Hamming I, Sadaghiani S, Steinmetz OM,
Meyer-Schwesinger C, Fehr S, et al. Antihypertensive
therapy upregulates renin and (pro)renin receptor in the clipped
kidney of Goldblatt hypertensive rats. Kidney Int.
2007;72:725-30.
27.
Badila, Elisabeta, Tintea, Emma. How to manage
renovascular hypertension. E-Journal ESC Council for Cardiology
Practice;13. Available from:
https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/How-to-manage-renovascular-hypertension.
Accessed August 25, 2019.
28.
Vo NJ, Hammelman BD, Racadio JM, Strife CF, Johnson ND,
Racadio JM. Anatomic distribution of renal artery stenosis in
children: Implications for imaging. Pediatr Radiol.
2006;36:1032-6.
29.
Krishna A, Kumar O, Singh MK. Renovascular hypertension:
A review article. Clinical Queries: Nephrology. 2013;2:38-43.
30.
Dillman JR, Smith EA, Coley BD. Ultrasound imaging of
renin-mediated hypertension. Pediatr Radiol. 2017;47:1116-24.
31.
Drieghe B, Madaric J, Sarno G, Manoharan G, Bartunek J,
Heyndrickx GR, et al. Assessment of renal artery
stenosis: side-by-side comparison of angiography and duplex
ultrasound with pressure gradient measurements. Eur Heart J.
2008;29:517-24.
32.
Schäberle W, Leyerer L, Schierling W, Pfister K.
Ultrasound diagnostics of renal artery stenosis: Stenosis
criteria, CEUS and recurrent in-stent stenosis. Gefasschirurgie.
2016;21:4-13.
33.
Trautmann A, Roebuck DJ, McLaren CA, Brennan E, Marks SD,
Tullus K. Non-invasive imaging cannot replace formal angiography
in the diagnosis of renovascular hypertension. Pediatr Nephrol.
2017;32:495-502.
34.
Zhang HL, Sos TA, Winchester PA, Gao J, Prince MR. Renal
artery stenosis: Imaging options, pitfalls, and concerns. Prog
Cardiovasc Dis. 2009;52:209-19.
35. Tan KT, van Beek EJR,
Brown PWG, van Delden OM, Tijssen J, Ramsay LE. Magnetic
resonance angiography for the diagnosis of renal artery
stenosis: A meta-analysis. Clin Radiol. 2002;57:617-24.
36.
Hansen NJ, Kaza RK, Maturen KE, Liu PS, Platt JF.
Evaluation of low-dose CT angiography with model-based iterative
reconstruction after endovascular aneurysm repair of a thoracic
or abdominal aortic aneurysm. AJR Am J Roentgenol.
2014;202:648-55.
37.
Roebuck DJ. Pediatric interventional radiology. Imaging.
2001;13:302-20.
38.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA,
Halperin JL, et al. ACC/AHA Guidelines for the Management
of Patients with Peripheral Arterial Disease (lower extremity,
renal, mesenteric, and abdominal aortic): A collaborative report
from the American Associations for Vascular Surgery/Society for
Vascular Surgery, Society for Cardiovascular Angiography and
Interventions, Society for Vascular Medicine and Biology,
Society of Interventional Radiology, and the ACC/AHA Task Force
on Practice Guidelines (writing committee to develop guidelines
for the management of patients with peripheral arterial disease)
– Summary of Recommendations. J Vasc Interv Radiol.
2006;17:1383-97.
39.
Seeman T, Dostálek L, Gilík J. Control of hypertension in
treated children and its association with target organ damage.
Am J Hypertens. 2012;25:389-95.
40.
Piercy KT, Hundley JC, Stafford JM, Craven TE, Nagaraj
SK, Dean RH, et al. Renovascular disease in children and
adolescents. J Vasc Surg. 2005;41:973-82.
41.
Lacombe M. Surgical treatment of renovascular
hypertension in children. Eur J Vasc Endovasc Surg.
2011;41:770-7.
42.
Towbin RB, Pelchovitz DJ, Cahill AM, Baskin KM, Meyers
KEC, Kaplan BS, et al. Cutting balloon angioplasty in
children with resistant renal artery stenosis. J Vasc Interv
Radiol. 2007;18:663-9.
43.
Srinivasan A, Krishnamurthy G, Fontalvo-Herazo L, Nijs E,
Keller MS, Meyers KE, et al. Angioplasty for renal artery
stenosis in pediatric patients: An 11-year retrospective
experience. J Vasc Interv Radiol. 2010;21:1672-80.
44.
Meyers KE, Cahill AM, Sethna C. Interventions for
pediatric renovascular hypertension. Curr Hypertens Rep.
2014;16:422.
45.
O’Neill JA. Long-term outcome with surgical treatment of
renovascular hypertension. J Pediatr Surg. 1998;33:
106-11.
46.
Stanley JC, Criado E, Upchurch GR, Brophy PD, Cho KJ,
Rectenwald JE, et al. Pediatric renovascular
hypertension: 132 primary and 30 secondary operations in 97
children. J Vasc Surg. 2006;44:1219-28.
47. Hegde S, Coulthard MG.
Follow-up of early unilateral nephrectomy for hypertension. Arch
Dis Child Fetal Neonatal Ed. 2007;92:F305-6.

