The 2019 novel
coronavirus (COVID-19) pneumonia, reported in Wuhan (Hubei
Province, China) since late 2019, has garnered intense attention
worldwide [1]. The World Health Organization has declared this
outbreak as a pandemic. While COVID-19 is usually common in
middle-aged or elderly people, the incidence of COVID-19 is rare
in children, and children have mild clinical symptoms [2]. Chest
computed tomography (CT) can identify infected lesions,
indicating viral pneumonia, which plays an irreplaceable role in
the screening of COVID-19. There are only limited data available
regarding the typical chest CT imaging findings of COVID-19 in
children [2]. In this study, we retrospectively evaluated
radiographic features of chest CT and clinical features in
children with confirmed COVID-19.
METHODS
This study was approved by the Medical Research Ethics
Committee of our institution. The requirement for patients’
informed consent was waived due to the retrospective nature of
the study. From January 16, 2020 to March14, 2020, a search of
the electronic system and Picture archiving and communication
system (PACS) was performed in our department. All pediatric
patients with suspected/proven COVID-19 were being routinely
subjected to CT chest at our center.The inclusion criteria were:
(i) epidemiological history: either travel/residence
history in Wuhan or exposure history to patients with fever from
Wuhan suffering from respiratory symptoms within 14 days before
the onset of illness; and (ii) laboratory diagnosis:
positive detection of COVID-19 in throat swabs or lower
respiratory tract by real-time fluorescence polymerase chain
reaction (Shanghai ZJ Bio-Tech Co, Ltd, Shanghai, China).
The following clinical data of the patients were collected and
assessed: sex, age, pharyngeal discomfort, cough, expectoration,
chest congestion, myalgia and abdominal pain or diarrhea.
Information regarding the physical examination at admission was
evaluated, including the heart rate, body temperature,
respiratory rate and blood pressure. Moreover, the laboratory
data were also assessed, including total and differential
leukocyte, erythrocyte sedimentation rate (ESR), procalcitonin,
and C-reactive protein (CRP) levels.
Imaging technique: All
patients completed non-contrast chest CT scans in a separate
examination room while the CT technician utilized secondary
protection. Chest CT images were obtained using a 16-row
multi-detector CT scanner (Siemens Somatom Sensation; Siemens,
Erlangen, Germany). The CT examination parameters were as
follows: 120 kVp, 140 mA, 5 mm collimation, 1.35:1 pitch, a
pulmonary kernel (B70f) and a mediastinal kernel (B30f),
reconstruction slice thickness of 1.0 mm, and high spatial
resolution algorithm. All the patients over the age of three
years were scanned in a supine position while holding their
breath at full inspiration, while children under the age of
three completed examinations while asleep (were not required to
hold their breath).
All chest CT scans were reviewed independently by two senior
radiologists, while they were blinded to the name and clinical
data of the patients. The two radiologists reached a consensus
about the lung abnormalities, and an agreement was reached by
discus-sion if the conclusions were different. All CT images
were viewed on both lung window (width, 1500 HU; level, 500 HU)
and mediastinal window (width, 350 HU; level, 40 HU) settings.
The major CT dimensions, including the presence of ground-glass
opacities, ‘crazy-paving sign’, consolidation, and mixed
ground-glass opacities and consolidation lesions, were fully
evaluated. The detailed definitions of the above features were
as per a previous publication [3,4]. The distribution of lung
abnormalities was recorded as predominantly sub-pleural
(involving mainly the peripheral one-third of the lung), central
(involving mainly the central one-third of the lung), mixed
(involving both sub-pleural and central regions), and diffuse
(continuous involvement without respect to lung segments)
according to a similar report [5]. The scattering patterns of
lesions (focal, multifocal and diffuse) were also classified.
The number of bilateral lung segments affected by pneumonia was
recorded simultaneously.
Statistical analyses: All statistical
analyses were conducted using Statistical Package for Social
Sciences software version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Records of 22 patients (12 males) were included in this study.
The mean (SD) age was 8 (6) years. The most prevalent presenting
symptoms were fever (14 cases, 64%) and cough (13 cases, 59%).
Two patients had no clinical symptoms or chest CT abnormalities;
however, they were in close contact with confirmed cases and had
positive results on a COVID-19 nucleic acid test. Chest CT scans
were obtained at mean (SD) 3 (3) days (range: 0-11) after the
onset of symptoms. Laboratory investigations showed that the
most frequent abnor-malities were mildly elevated CRP [mean
(SD)=11.2 (11.6)] and ESR values [mean (SD) = 18.8 (15.17)]
(Web Table I).
Table I Computed Tomography Chest findings in Pediatric Patients with COVID-19 (N=22)
|
Finding |
No. (%) |
|
Ground glass opacities |
3 (14) |
|
Crazy-paving sign |
2 (9) |
|
Consolidation |
7 (32) |
|
Ground glass opacities and consolidation |
8 (36) |
|
Lung region distribution | |
|
Unilateral |
5 (23) |
|
Bilateral |
15 (68) |
|
Subpleural |
10 (45) |
|
Central |
1 (5) |
|
Mixed |
9 (41) |
|
Lung lobe involved | |
|
Right upper lobe |
2 (9) |
|
Right lower lobe |
9 (41) |
|
Left upper lobe |
3 (14) |
|
Left upper lobe |
6 (27) |
|
*Lung segments involved |
3 (3) |
|
Distribution | |
|
Focal |
3 (14) |
|
Multifocal |
15 (68) |
|
Diffuse |
2 (9) |
|
Data represented as no. (%) or *mean (SD). Two patients had no chest CT abnormalities. |
Most patients showed bilateral lung involvement (15, 68%) (Table
I). Of the spatial distribution of all lesions, the
right lower lobe (9, 41%) was most commonly involved. The
average number of infected lung segments was three (range: 0-15)
for all patients, with less than three lung segments involved in
55% patients. The major CT abnormalities observed were mixed
ground glass opacities and consolidation lesions (8, 36%), or
consolidations alone (7, 32%) (Fig.1). In
addition, two children (9%) showed
a ‘crazy-paving sign’ characterized by reticular
interlobular septal thickening within patchy ground glass
opacities [6]. Multifocal lesions (15 cases, 68%) were most
common, and patients had lymph node enlargement.
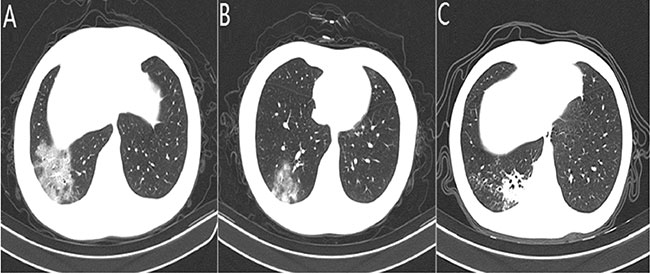 |
| Fig. 1 Computed tomography findings
in children with coronavirus-19 (COVID-19) pneumonia:
(a) ground glass opacities; (b) consolidation; and (c)
ground glass opacities and consolidation. |
DISCUSSION
The novel
coronavirus related to the MERS and SARS coronaviruses [7,8] has
now spread to become a pandemic, with wide-ranging effects.We
found that typical clinical symptoms were similar to those of
other types of coronavirus infections, such as SARS and MERS
[9-11]. CT chest findings were noted to be characteristic and
have been detailed.
There is usually a certain time interval between the onset of
symptoms and hospitalization of COVID-19 patients. In our study,
the time interval between the first chest CT examination and the
onset was 0 to 11 days. An initial negative result of chest CT
examination may occur (2 cases, 9%), but lung abnormalities
could be discovered among most of the patients.Most of the
patients had mild symptoms as well as temperature elevation, but
their lung manifestations were relatively obvious. In contrast
tothose in bacterial pneumonia [4], lung lesions showed similar
imaging features to other viral pneumonias, mainly ground glass
opacities, extensive interlobular septal thickening and patchy
consolidation. In the early stage, COVID-19 reflects mainly
interstitial lung damage, such as thickening of the interlobular
septa and the presence of ground glass opacities. Alveolar
edema, exudation and bleeding can be manifested in different
degrees of ground glass opacities on CT images, as inflammation
involves the alveoli.In more than one-third of cases, abnormal
lung manifestations were presented as a mixture of GGOs and
consolidations, implying a rapid progression of pneumonia, which
is probably due to a lower immune response in children than in
adults. In severe cases, owing to the collapse of alveoli or
massive infiltration of inflammatory cells, lung parenchymal
injury may occur, presentingas lung consolidation. Although more
detailed pathological changes of COVID-19 need to be further
studied, research has shown that angiotensin-converting enzyme 2
(ACE2) is an important receptor of the SARS-CoV-2 surface spike
protein domain, which is similar to the case for SARS-CoV. Given
that it is highly contagious, its affinity may be greater than
that of SARS-CoV [12]. Human ACE2 receptors are abundantly
expressed in type II alveolar epithelial cells. The outer bands
of the lungs and the sub-pleural space are dense areas where
terminal bronchi are dilated to form alveolar ducts, which can
also explain the characteristics of lesion distribution from an
anatomical perspective. In addition, thickened small blood
vessel shadows and faint shadows surrounding the nodules are
also characteristic in some reports.
Globally, similar outbreaks of respiratory diseases have also
been observed for SARS and MERS, and those causative pathogens
belong to the beta-coronavirus family [13]. There is a certain
similarity of chest CT imaging features between SARS and
COVID-19 [14]. For instance, both GGOs and consolidations are
dominant and concentrate mainly in bilateral sub-pleural areas;
otherwise, cavities, pleural effusion and enlarged lymph nodes
are rare. However, SARS had more severe interstitial fibrosis
during the absorption phase of pneumonia. After discharge, CT
images of SARS patients still showed thickening of the lobular
septum, subpleural and distal bronchiolar dilatation, and
honeycomb changes [15]. The lung lesions of SARS progressed more
rapidly and were termed white out or ‘white lung’ because
consolidation and coalescing infiltrates pervaded the lungs,
leaving few recognized air spaces. The COVID-19 patients in this
study did not show ‘white lung’, although we observed lesions
involving 15 lung segments in one case, and the distribution of
lesions was mainly sub-pleural. Furthermore, we note that 55% of
cases involved less than three lung segments, which is different
from previous reports of adult patients. This finding suggests
that COVID-19 has a mild inflammatory infiltration in children,
which indicates that they are more likely to recover than adults
after symptomatic treatment, and the specific mechanism needs to
be further studied.
This study has several limitations. First, the sample size of
this study is small because the incidence of children with
COVID-19 is not high. Including additional cases could have
improved the recognition of image features of COVID-19 in
children. Second, longitudinal studies on follow-up CT changes
during treatment in children need to be carried out. These
studies can reflect the course of disease development and
pathological changes and may provide valuable experience for
future treatment and rehabilitation.
In summary, we found that common chest CT findings in COVID-19
in children include multiple mixed ground glass opacities and
consolidation lesions in both lungs, with mostly sub-pleural
distribution. The ‘crazy-paving sign’ was found in a few cases,
and the number of lung segments involved was small, with an
average of three. More data on this aspect will assist
clinicians in diagnosis and management of COVID-19 in children.
Contributors:
All authors have contributed, designed and approved the study.
Funding:
None; Competing interest: None stated
Ethical approval:
Medical Research Ethics Committee of Yichang Central People’s
Hospital, Yichang, China.
|
What This StudyAdds? |
• Chest computed tomography findings and
clinical features of pediatric patients with COVID-19.
|
REFERENCES
1. World Health
Organization. Novelcoronavirus-China. Jan12,2020. Available
from: http://www.who.int/csr/
don/12-january-2020-novel-coronavirus-china/en/. Accessed
March14, 2020.
2. Chan JF, Yuan S, Kok KH,
To KK, Chu H, Yang J, et al. A familial cluster of
pneumonia associated with the 2019 novel coronavirus indicating
person-to-person transmission: A study of a family cluster.
Lancet. 2020; 395: 514-23.
3. Koo HJ, Lim S, Choe J,
Choi SH, Sung H, Do KH. Radiographic and CT features of viral
pneumonia. Radiographics. 2018;38:719-39.
4. Wu J, Wu X, Zeng W, Guo
D, Fang Z, Chen L, et al. Chest CT findings in patients
with coronavirus disease 2019
and its relationship with clinical features. Invest
Radiol. 2020;10.1097/RLI.0000000000000670. Available form
https://journals.lww.com/investigativeradiology/Abstract/publishahead/Chest_CT_Findings_in_Patients_with_
Corona_Virus.98835.aspx. Accessed March 20, 2020.
5. Ooi GC, Khong PL, Müller
NL, Yiu WC, Zhou LJ, Ho JC, et al. Severeacute
respiratory syndrome: temporal lung changes at thin-section CT
in 30 patients. Radiology. 2004;230: 836-44.
6. Wong KT, Antonio GE, Hui
Hui DS, Lee N, Yuen EH, Wu A, et al. Thin-section CT of
severe acute respiratory syndrome: evaluation of 73 patients
exposed to or with the Disease. Radiology. 2003;228:395-400.
7. Cohen J, Normile D. New
SARS-like virus in China triggers alarm. Science. 2020;367:
234-5.
8. Zhu N, Zhang D, Wang W,
Li X, Yang B, Song J, et al. A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med.
2020;382:727-33.
9. Lee N, Hui D, Wu A, Chan
P, Cameron P, Joynt GM, et al. A major outbreak of severe
acute respiratory syndrome in Hong Kong. N Engl J Med.
2033;348:1986-94.
10. Assiri A, Al-Tawfiq JA,
Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al.
Epidemiological, demo-graphic, and clinical characteristics of
47 cases of Middle East respiratory syndrome coronavirus disease
from Saudi Arabia: A descriptive study. Lancet Infect Dis. 2013;
13:752-61.
11. Müller NL, Ooi GC,
Khong PL, Zhou LJ, Tsang KW, Nicolaou S. High-resolution CT
findings of severe acute respiratory syndrome at presentation
and after admission. Am J Roentgenol. 2004;182:9-44.
12. Liu Y, Gayle AA,
Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19
is higher compared to SARS coronavirus. J Travel Med. 2020.
Available form:
https://academic.oup.com/jtm/article/27/2/taaa021/5735319.
Accessed March 20, 2020.
13. Zhang L, Liu Y.
Potential interventions for novel coronavirus in china: A
systemic review. J Med Virol. 2020. [Epub ahead of print].
Available form
https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.25707.
Accessed March 20, 2020.
14. Chung M, Bernheim A,
Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging
features of 2019 novel coronavirus (2019-nCoV).Radiology. 2020.
[Epub ahead of print] Available form
https://pubs.rsna.org/doi/pdf/10.1148/radiol.2020200230.
Accessed March 20, 2020.
15.Xiangke D, Wanjiang Y, Silun W. Preliminary
analysis of clinical images of SARS (in Chinese). Chinese J
Radiol. 2003;37:780-3.

