test was used to determine statistical significance with regard to
heterogeneity.
We performed statistical analysis using the Revman
software. Pooled estimates of the evaluated outcome measures were
calculated by the generic inverse variance method. Pooled WMD and SMD
were calculated as per standard recommendations [8]. We expected
variation in studies with respect to populations, interventions,
comparators, outcomes and settings, and thus used the random-effects
model. If it was not possible to synthesize the data from the included
studies, we provided a narrative synthesis of the results. The data were
finally synthesized as a ‘Summary of findings’ table. For each outcome,
quality assessment of the results was also carried out using the GRADE
approach [10], which specifies four levels of quality (high, moderate,
low and very low) where the highest quality rating is for a body of
evidence based on randomized trials. We planned to explore the following
differences in effect for ‘length’, by subgroup analyses: (i)
supplementation method (medicinal versus fortification); (ii)
supplement compound; (iii) study population from South Asia; (iv)
dose of zinc (mg); (v) duration of supplementation; (vi)
compliance estimation (directly observed or replacement versus others);
(vii) baseline zinc levels; and (viii) baseline prevalence
of stunting. We chose length-for-age Z-score as the variable for
subgroup analysis, as it is an age-independent parameter and more
important from public health perspective. We could not do the subgroup
analysis for the first parameter (supplementation method) as all studies
had used medicinal supplementation.
Results
The search output from various databases is detailed
in Web Appendix 1, and the results are summarized in
Fig. 1. We screened 3886 records, of which 237 were
potentially eligible. Of these, 147 references were excluded and 91
publications (63 studies) were included in the final analyses
[3,11-100]. These studies (5 cluster RCTs and 58 RCTs) incorporating
data on 27372 children were included in the final analysis (Web
Table I). Twenty-eight (44%) of the included trials were
conducted in Asia (17 from South Asia), 16 trials were conducted in
Africa and 19 in Latin America. The details of study location,
intervention and outcomes are summarized in Web
Table I.
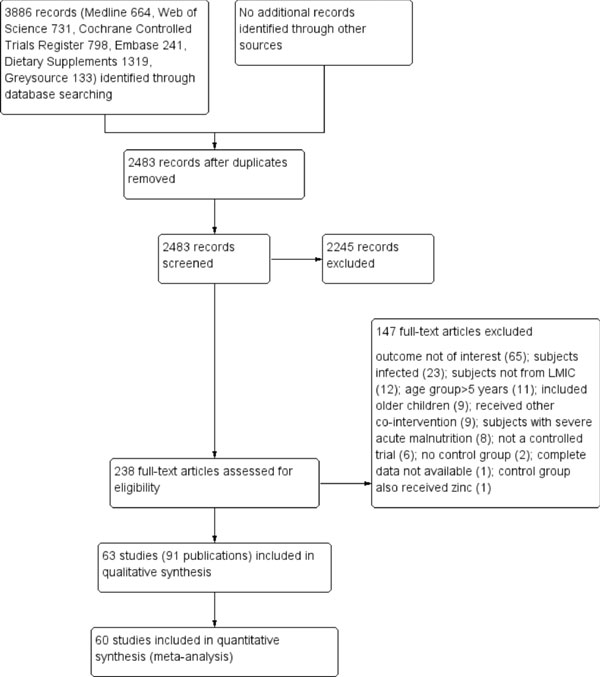 |
|
Fig. 1 The PRISMA flow chart.
|
Web Fig.
1 and Web Fig. 2
summarize the Risk of Bias for the included studies. The risk of bias
for the 55 trials was low for random sequence generation. It was
considered to be high for two trials and unclear for the remaining six
studies. The risk of bias for allocation concealment was judged to be
low in 39, unclear in 21 and high in 3 studies. Blinding of participants
and research personnel was at unclear risk in 5, at high risk in 2
studies and low risk in 56 trials. The risk of blinding of outcome
assessment was considered low for 34 trials, unclear for 27 and high for
2 trials. The risk of bias for attrition was judged to be high for 32
trials, unclear for 2 trials because of no information available, and
low for remaining 29 trials. In the five cluster randomized trials, two
studies were considered to be at unclear risk for incorrect analysis and
one trial for baseline imbalance. Seven trials were judged to be having
other potential causes of bias, including baseline imbalance of groups
(3), formula milk use (2) and protocol deviations related to key
intervention (2).
Effects of Interventions
Height/Length (Web Appendix 3A):
Twenty-nine trials reported data on height-for-age Z-score (HAZ) in the
study participants. Quantitative synthesis from 25 trials (Fig.
2) revealed no evidence of effect of zinc supplementation on HAZ
(9165 participants; MD= 0.00; 95% CI -0.07, 0.07; P=0.98;
Moderate Quality Evidence) in comparison to controls, with significant
heterogeneity between trials (I² = 57%; P<0.001). In subset
analysis to explore heterogeneity, the dose of zinc and duration of zinc
supplementation were important predictors of heterogeneity. Supplement
compound, location in South Asia, compliance estimation, baseline serum
zinc levels, baseline prevalence of stunting and baseline HAZ did not
predict heterogeneity. Thirteen trials studied the effect of zinc
supplementation on change in HAZ. On quantitative synthesis in 8852
participants, the MD for change in HAZ was 0.11 (95% CI -0.00, 0.21;
P=0.05; Moderate Quality Evidence; Fig. 3) with
substantial heterogeneity between trials (I² = 94%; P<0.001).
Twenty-one trials reported the effect of zinc supplementation on
length/height at the end of supplementation period. On quantitative
synthesis from 19 trials, there was no evidence of effect on
length/height (6303 participants; MD= 1.18 cm; 95% CI -0.63, 2.99 cm,
P=0.20; Moderate Quality Evidence; considerable heterogeneity,
I²=99%; Fig. 4) with zinc supplementation as compared to
controls. Twenty-six trials reported the effect of zinc supplementation
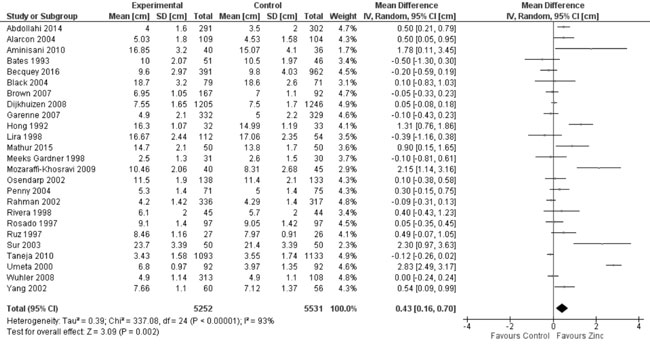
(vs. controls) on change in length/height (cm) from baseline to
the end of supplementation period. In 25 of these trials with 10783
participants, the pooled change in length/height with zinc
supplementation as compared to controls was 0.43 cm (95% CI 0.16, 0.70,
P=0.002; considerable hetero-geneity, I²=93%; Moderate Quality
Evidence; Fig. 5). Funnel plots of all height-related
outcomes showed no evidence of publication bias (Web
Fig. 3a to 3d).
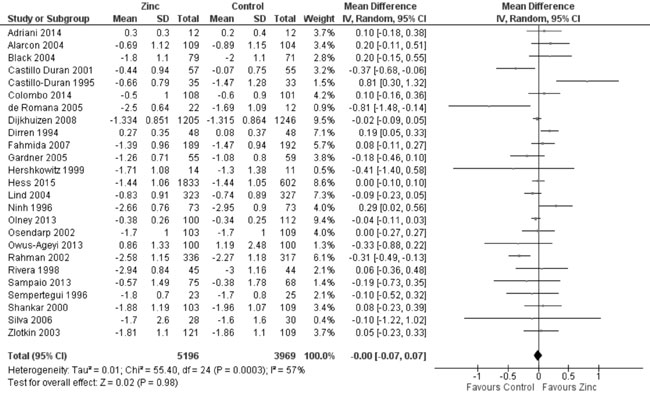 |
|
Fig. 2 Forest plot of effect of zinc
supplementation on height-for-age Z scores.
|
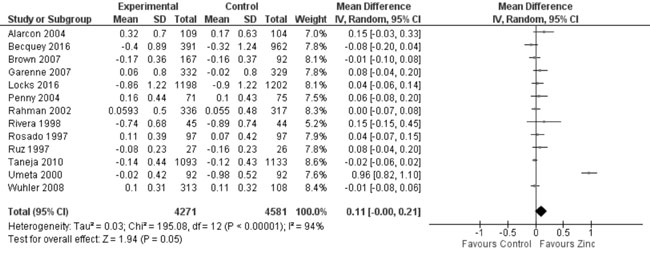 |
|
Fig. 3 Forest plot of effect of zinc
supplementation on change in height-for-age Z scores.
|
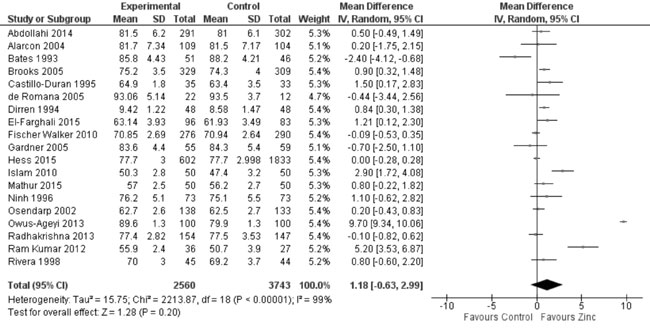 |
|
Fig. 4 Forest plot of effect of zinc
supplementation on height/length at the end of supplementation
period.
|
 |
|
Fig. 5 Forest plot of effect of zinc
supplementation on change in height/length.
|
Weight (Web Appendix 3B):
Twenty-five trials reported data on weight-for-age Z-score (WAZ) in the
study participants. In 23 trials on 9033 participants (Fig. 6),
the mean difference in WAZ was 0.05 (95% CI -0.03, 0.13; P=0.19;
Moderate Quality Evidence; substantial heterogeneity, I²=75%) between
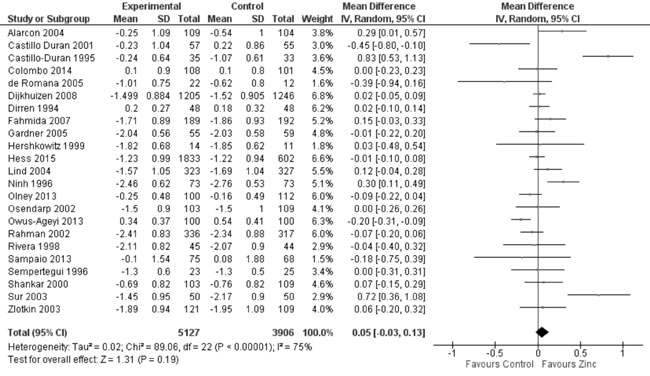
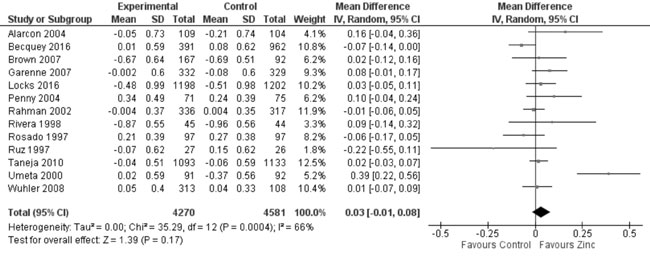
zinc supplemented and control group. Thirteen trials studied the effect
of zinc supplementation on change in WAZ from baseline. Quantitative
synthesis from these trials (Fig. 7) showed no evidence of
effect on change in WAZ with zinc supplementation in comparison to
controls (8851 study participants; MD= 0.03; 95% CI -0.01, 0.08; P=0.17;
Moderate Quality Evidence; substantial heterogeneity, I²=66%). Weight at
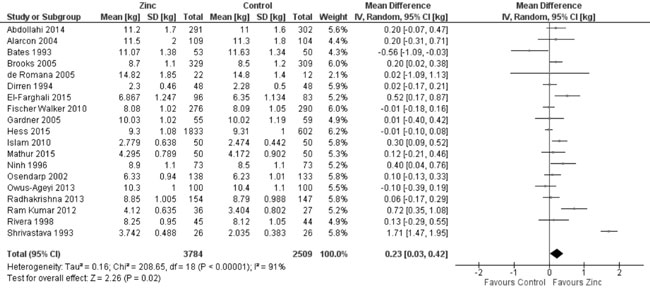
the end of the supplementation period was reported in 20 studies.
Quantitative synthesis from 19 of these trials (Fig. 8)
showed positive effect of zinc supplementation on weight as compared to
control population (8851 study participants; MD= 0.23 kg; 95% CI 0.03,
0.42; P=0.02; Moderate Quality Evidence). Twenty-three trials
reported on change in weight (kg) from baseline to the end of
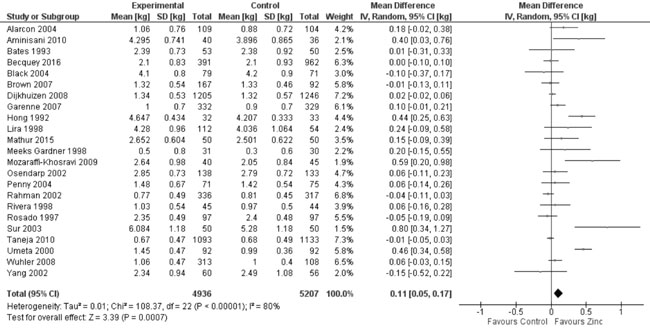
supplementation period. Quantitative synthesis (Fig. 9)
revealed a positive effect (10143 participants; MD=0.11 kg; 95% CI 0.05,
0.17; P<0.001; Moderate Quality Evidence) of zinc supplementation
in comparison to controls. There was significant heterogeneity between
trials comparing weight parameters between the two groups, and funnel
pots showed no evidence of publication bias (figures not shown).
 |
|
Fig. 6 Forest plot of effect of zinc
supplementation on weight-for-age Z scores.
|
 |
|
Fig. 7 Forest plot of effect of zinc
supplementation on change in weight-for-age Z scores.
|
 |
|
Fig. 8 Forest plot of effect of zinc
supplementation on weight at the end of supplementation period.
|
 |
|
Fig. 9 Forest plot of effect of zinc
supplementation on change in weight.
|
Weight-for-height (Web Appendix 3C):
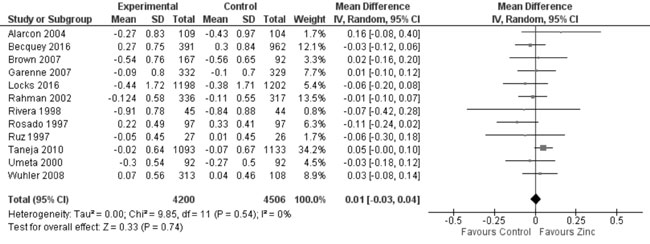
In 22 trials reporting data on weight-for-height Z-score (WHZ), there
was no evidence of effect of zinc supplementation on WHZ in comparison
to controls (19 trials; 8392 study participants; MD=0.03; 95% CI -0.02,
0.08; P=0.21; Moderate Quality Evidence; considerable
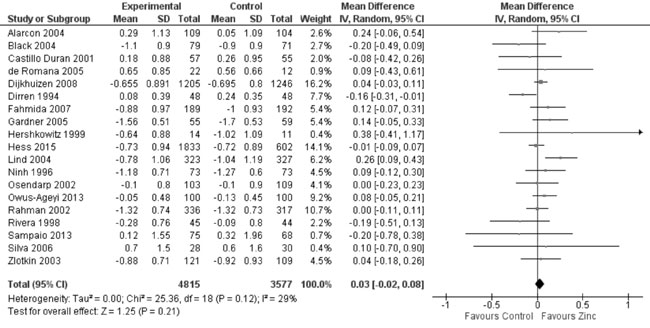
heterogeneity, I²=91%; Fig. 10). In 12 trials evaluating
the change in weight from height Z-scores, there was no evidence of
effect on change in WHZ with zinc supplementation as against controls
(8706 study participants; MD= 0.01; 95% CI -0.03, 0.04; P=0.74;
Moderate Quality Evidence; substantial heterogeneity, I²=80%; Fig.
11). There was no evidence of publication bias on examining the
funnel plots (figures not shown).
 |
|
Fig. 10 Forest plot of effect of zinc
supplementation on weight-for-height Z scores at the end of
supplementation period.
|
 |
|
Fig. 11 Forest plot of effect of zinc
supplementation on change in weight-for-height Z scores.
|
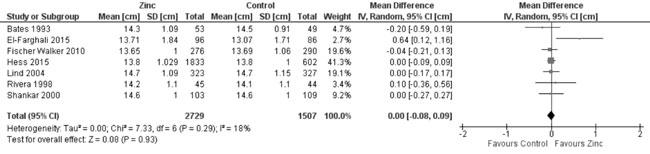
MUAC (Web Appendix 3C): In 7 trials
evaluating MUAC, there was no effect of zinc supplementation (vs.
controls) on MUAC (4236 participants; MD = 0.0 cm; 95% CI -0.08, 0.09;
P=0.93; Moderate Quality Evidence) with no significant
heterogeneity between trials (I² = 18%; P=0.29) (Fig.
12). There was moderate quality evidence of little increase in the
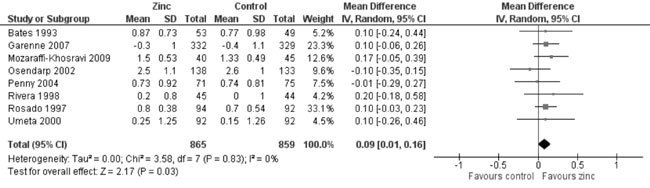
change in MUAC from baseline (8 trials; 1724 participants; MD = 0.09 cm;
95% CI 0.01, 0.16; P=0.03; no heterogeneity, I²=0%) by zinc
supplementation in comparison to controls (Fig. 13).
 |
|
Fig. 12 Forest plot of effect of zinc
supplementation on mid upper arm circumference at the end of
supplementation period.
|
 |
|
Fig. 13 Forest plot of effect of zinc
supplementation on change in mid upper arm circumference.
|
Head circumference (Web Appendix 3D):
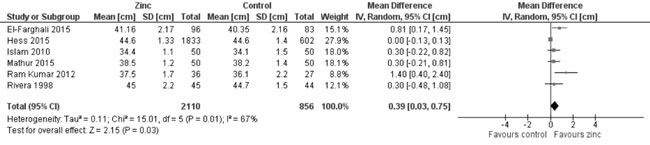
In quantitative synthesis from six trials (Fig. 14) showed
higher head circumference in zinc supplemented group as against control
group (2966 participants; MD= 0.39 cm; 95% CI 0.03, 0.75; P=0.03;
Moderate Quality Evidence; substantial heterogeneity, I²=67%). However,
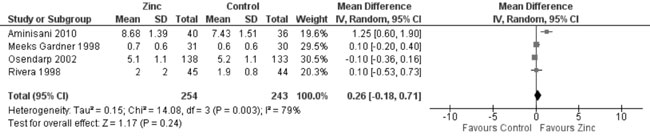
change in head circumference was not different in the zinc supplemented
and placebo groups (4 trials; 497 participants; MD = 0.26 cm; 95% CI
-0.18, 0.71: P=0.24; Moderate Quality Evidence; substantial
heterogeneity, I²=79%) (Fig. 15).
 |
|
Fig. 14 Forest plot of effect of zinc
supplementation on head circumference at the end of
supplementation period.
|
 |
|
Fig. 15 Forest plot of effect of zinc
supplementation on change in head circumference.
|
Nutritional Status (Web Appendix 3D):
In trials reporting on stunting, underweight or wasting, funnel plots
did not show any evidence of publication bias (figures not shown).
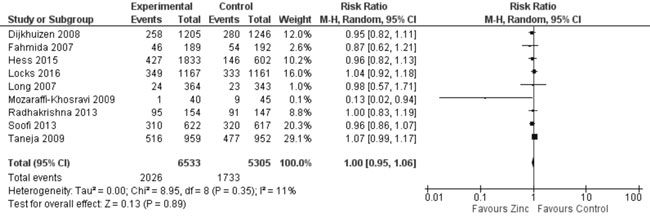
Quantitative synthesis from nine trials (Fig. 16) showed
no effect on stunting (11838 participants; RR= 1.0; 95% CI 0.95, 1.06;
P=0.89; Moderate Quality Evidence; no significant heterogeneity,
I²=11%) with zinc supplementation in comparison to controls. In 7 trials
reporting on the prevalence of underweight children, quantitative
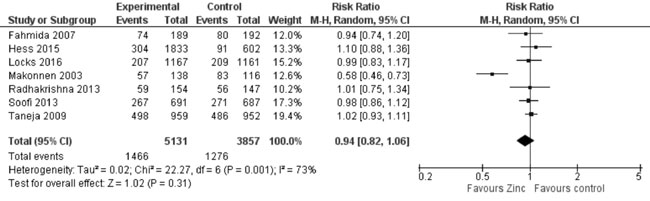
synthesis (Fig. 17) showed no effect of zinc
supplementation (vs. controls) on underweight (8988 participants;
RR= 0.94; 95% CI 0.82, 1.06; P=0.31; Moderate Quality Evidence;
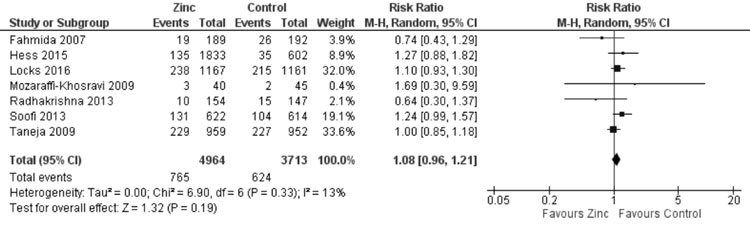
substantial heterogeneity, I²=73%). Quantitative synthesis (Fig.
18) from seven trials showed no effect of zinc supplemen- tation on
wasting (8677 participants; RR= 1.08; 95% CI 0.96, 1.21; P=0.19;
Moderate Quality Evidence) with no significant heterogeneity (I²=13%,
P=0.33).
 |
|
Fig. 16 Forest plot of effect of zinc
supplementation on stunting.
|
 |
|
Fig. 17 Forest plot of effect of zinc
supplementation on underweight.
|
 |
|
Fig. 18 Forest plot of effect of zinc
supplementation on wasting.
|
Discussion
In this systematic review of 63 trials incorporating
data on 27372 children, there was no evidence of any difference in the
final length/height for age or Z scores or change in
length/height-for-age Z scores at the end of the supplementation period
with zinc or placebo/no intervention, but studies assessing the change
in length/height showed slight benefit with zinc supplementation. In
addition, there was marginal increase in weight of children receiving
zinc supplementation in comparison to placebo, but it did not affect
weight-for-age or weight-for-height Z scores. There was a marginal
positive effect on the change in MUAC from baseline. Zinc supplemented
children also had a slightly higher head circumference at the end of
supplementation period, but there was no evidence of effect on change in
head circumference. Moreover, there was no evidence of a beneficial
effect on prevalence of wasting, stunting or underweight at the end of
supplementation period.
All included studies involved children under five
years from LMICs. This is a population that is likely to have poor zinc
nutriture and, therefore, benefit more from zinc supplementation. A
large number of trials were available from varied geographical settings
(28 from Asia, 16 from Africa, and 19 from Latin America), conducted in
different age groups and in different population settings (ranging from
tertiary level medical institutions to community studies in urban slums
and rural communities). Control groups in most trials were comparable
with intervention groups at baseline. Thus any observed effects, or lack
thereof, in the intervention groups are more likely to be attributable
to zinc supplementation. We, therefore, believe that the evidence from
this review is largely applicable to real-life situations among
under-five children in LMICs.
Most studies in this systematic review had a low risk
of bias for key parameters, including sequence generation, allocation
concealment and blinding. Also given the large number of studies
available for most outcomes, the certainty of evidence is reasonable
(moderate quality) for most of the important outcomes, and this review
is likely to provide a good indication of the likely effect. The review
was conducted by following the guidelines laid down in the Cochrane
Handbook for Systematic Review [8], and this is likely to eliminate most
sources of bias and identify the remaining. In some studies,
anthropometric measurements were not available as the results were
either depicted only in graphs or summary statistics, which is a
potential source of bias. However, this is unlikely to affect the
overall direction of results as narrative synthesis from these few
studies was broadly in agreement with quantitative synthesis from this
systematic review.
The earliest systematic review on this topic by
Brown, et al. [6] included 33 studies, and reported a meaningful
positive effects of zinc supplementation in height-for-age Z-score and
weight-for-age Z-score without significant effect on weight-for-height
indices. However, this review also included older children, besides
being not restricted to LMICs. Ramakrishnan, et al. [7] reviewed
43 trials, and reported marginal benefits in terms of change in HAZ, WAZ
and WHZ. Imdad, et al. [101] reported a significant positive
effect of zinc supplementation on height gain in the developing
countries, but studies providing other micronutrients in addition to
zinc were also included. Mayo-Wilson, et al. [102], in a review
of 50 studies (including children from all countries), showed no
evidence of difference in height or stunting with little increase in
weight and weight-to-height ratio. A very recent systematic review [103]
evaluated effect of zinc supplementation provided during antenatal
period or during childhood, and reported slightly increased height,
weight and weight-for-age Z-score, but no effect on height-for-age
Z-score, weight-for-height Z score, stunting, underweight or wasting,
with supplementation provided after birth. In comparison to this review,
our review focussed on LMIC where the problem of zinc deficiency is
considered a major public health problem. In comparison to the review by
Liu, et al. [103], the present review included more trials (63
vs. 54), probably because of a wide variety of database search and
inclusion of trials with shorter (<3 mo) duration of supplementation.
However, these results are broadly in conformity with our findings;
marginal differences probably arise from variations in populations and
analytical methods.
Evidence from this review suggests that zinc
supplementation probably leads to little or no improvement in
anthropometric indices and malnutrition (stunting, underweight and
wasting) in children under five years of age from LMICs. Advocating zinc
supplementation as a public health measure to improve growth, therefore,
appears unjustified in these settings with scarce resources. Using high
stunting prevalence as an indicator of population-level zinc deficiency
is also questionable. However, as most studies in this review examined
the effects of medicinal supplementation with zinc, effect of
fortification of foods with zinc on growth needs to be evaluated in
pragmatic modes. Considering other potential benefits of zinc
supplementation, comprehensive evaluation of cost effectiveness,
including relative effects of medicinal and fortification routes, is
also desirable.
1. International Zinc Nutrition Consultative Group.
Assessment of the risk of zinc deficiency in populations and options for
its control. Food Nutr Bull. 2004;25:S91-204.
2. MacDonald RS. The role of zinc in growth and cell
proliferation. J Nutr. 2000;130:1500S-8S.
3. Ninh NX, Thissen JP, Collette L, Gerard G, Khoi
HH, Ketelslegers JM. Zinc supplementation increases growth and
circulating insulin-like growth factor I (IGF-I) in growth-retarded
Vietnamese children. Am J Clin Nutr. 1996;63:514-9.
4. O’Dell BL, Reeves PG. Zinc status and food intake.
In: Mills CF, editor(s). Zinc in Human Biology. Springer:Verlag,
1989. p.173-81.
5. Bhutta ZA, Black RE, Brown KH, Meeks-Gardner J,
Gore S, Hidayat A, et al. Prevention of diarrhea and pneumonia by
zinc supplementation in children in developing countries: pooled
analysis of randomized controlled trials. J Pediatr. 1999;135:689-97.
6. Brown KH, Peerson JM, Rivera J, Allen LH. Effect
of supplemental zinc on the growth and serum zinc concentrations of
prepubertal children: A meta-analysis of randomized controlled trials.
Am J Clin Nutr. 2002;75:1062-71.
7. Ramakrishnan U, Nguyen P, Martorell R. Effects of
micronutrients on growth of children under 5 y of age: Meta-analyses of
single and multiple nutrient interventions. Am J Clin Nutr.
2009;89:191-203.
8. Higgins JPT, Deeks JJ. Cochrane handbook for
systematic reviews of interventions version 5.1.0 [updated March 2011].
The Cochrane Collaboration, 2011.
9. Cochrane Community. Editorial and publishing
policy resource: Review manager (RevMan). Available from:
http://community.cochrane.org/editorial-and-publishing-policy-resource/information-technology/review-manager-revman.
Accessed September 19, 2017.
10. GRADEpro. GRADE’s software for Summary of
Findings Tables, Health Technology Assessment and Guidelines. Available
from: https://gradepro.org/. Accessed September 19, 2017.
11. Abdollahi M, Abdollahi Z, Fozouni F,
Bondarianzadeh D. Oral zinc supplementation positively affects linear
growth, but not weight, in children 6 24 months of age. Int J Prev Med.
2014;5:280-6.
12. Adriani M, Wirjatmadi B. The effect of adding
zinc to vitamin A on IGF-1, bone age and linear growth in stunted
children. J Trace Elements Med Biol. 2014;28:431-5.
13. Alarcon K, Kolsteren PW, Prada AM, Chian AM,
Velarde RE, Pecho IL, et al. Effects of separate delivery of zinc
or zinc and vitamin A on hemoglobin response, growth, and diarrhea in
young Peruvian children receiving iron therapy for anemia. Am J Clin
Nutr. 2004;80:1276-82.
14. Aminisani N, Barak M, Shamshirgaran SM. Effect of
zinc supplementation on growth of low birthweight infants aged 1–6 mo in
Ardabil, Iran. Indian J Pediatr. 2010;78:1239-43.
15. Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn
PG, Northrop-Clewes CA, et al. A trial of zinc supplementation in
young rural Gambian children. Br J Nutr. 1993;69:243-55.
16. Becquey E, Ouedraogo CT, Hess SY, Rouamba N,
Prince L, Ouédraogo JB, et al. Comparison of preventive and
therapeutic zinc supplementation in young children in Burkina Faso: A
cluster-randomized, community-based trial. J Nutr 2016;146:2058-66.
17. Becquey E, Ouedraogo CT, Hess SY, Rouamba N,
Prince L, Ouédraogo JB, et al. Comparison of preventive and
therapeutic zinc supplementation programs for young children in Burkina
Faso: A randomized, masked, community-based trial. Faseb J. 2013;27.
18. Black MM, Sazawal S, Black RE, Khosla S, Kumar J,
Menon V. Cognitive and motor development among small-for-gestational-age
infants: Impact of zinc supplementation, birth weight, and caregiving
practices. Pediatrics. 2004;113:1297-305.
19. Sazawal S, Black RE, Menon VP, Dinghra P,
Caulfield LE, Dhingra U, et al. Zinc supplementation in infants
born small for gestational age reduces mortality: A prospective,
randomized, controlled trial. Pediatrics. 2001;108:1280-6.
20. Brooks WA, Santosham M, Naheed A, Goswami D,
Wahed MA, Diener-West M, et al. Effect of weekly zinc supplements
on incidence of pneumonia and diarrhoea in children younger than 2 years
in an urban, low-income population in Bangladesh: randomised controlled
trial. Lancet. 2005;366:999-1004.
21. Arsenault JE, Havel PJ, de Romana DL, Penny ME,
Van Loan MD, Brown KH. Longitudinal measures of circulating leptin and
ghrelin concentrations are associated with the growth of young Peruvian
children but are not affected by zinc supplementation. Am J Clin Nutr.
2007;86:1111-9.
22. Arsenault JE, de Romana DL, Penny ME, Van Loan
MD, Brown KH. Additional zinc delivered in a liquid supplement, but not
in a fortified porridge, increased fat-free mass accrual among young
Peruvian children with mild-to-moderate stunting. J Nutr.
2008;138:108-14.
23. Brown KH, de Romana DL, Arsenault JE, Peerson JM,
Penny ME. Comparison of the effects of zinc delivered in a fortified
food or a liquid supplement on the growth, morbidity, and plasma zinc
concentrations of young Peruvian children. Am J Clin Nutr.
2007;85:538-47.
24. Castillo-Duran C, Rodriguez A, Venegas G, Alvarez
P, Icaza G. Zinc supplementation and growth of infants born small for
gestational age. J Pediatr. 1995;127:206-11.
25. Castillo-Durán C, Perales CG, Hertrampf ED, Marín
VB, Rivera FA, Icaza G. Effect of zinc supplementation on development
and growth of Chilean infants. J Pediatr. 2001;138:229-35.
26. Castillo-Duran C, Hertrampf ED, Ruz MO, Torreion
CS, Salazar G. Controlled trial of zinc supplementation on growth and
body composition in Chilean children from low income groups. Pediatr
Res. 2002;51:188A.
27. Chhagan MK, den Broeck JV, Luabeya KA, Mpontshane
N, Tomkins A, Bennish ML. Effect on longitudinal growth and anemia of
zinc or multiple micronutrients added to vitamin A: A randomized
controlled trial in children aged 6-24 months. BMC Public Health.
2010;10:145.
28. Caulfield L, Zavaleta N, Chen P, Colombo J,
Kannass K. Mineral status of non-anemic Peruvian infants taking an iron
and copper syrup with or without zinc from 6 to 18 months of age: A
randomized controlled trial. Nutrition. 2013;29:1336-41.
29. Colombo J, Caulfield LE, Kannass KN, Albornoz C,
Lazarte F, Zavaleta N. Six months of zinc supplementation affects
measures of attention in Peruvian infants at 12 months of age. Faseb J.
2011;25:.
30. Colombo J, Zavaleta N, Kannas KN, Lazarte F,
Albornoz C, Kapa LL, et al. Zinc supplementation sustained
normative neurodevelopment in a randomized, controlled trial of Peruvian
infants aged 6–18 months. J Nutr. 2014;144:1298-1305.
31. de Romana DL, Salazar M, Hambidge KM, Penny ME,
Peerson JM, Krebs NF, et al. Longitudinal measurements of zinc
absorption in Peruvian children consuming wheat products fortified with
iron only or iron and 1 of 2 amounts of zinc. Am J Clin Nutr.
2005;81:637-47.
32. Berger J, Ninh NX, Khan NC, Nhien NV, Lien DK,
Trung NQ, et al. Efficacy of combined iron and zinc
supplementation on micronutrient status and growth in Vietnamese
infants. Eur J Clin Nutr. 2006;60:443-54.
33. Dijkhuizen MA, Winichagoon P, Wieringa FT,
Wasantwisut E, Utomo B, Ninh NX, et al. Zinc supplementation
improved length growth only in anemic infants in a multi-country trial
of iron and zinc supplementation in South-East Asia. J Nutr.
2008;238:1969-75.
34. Pongcharoen T, DiGirolamo AM, Ramakrishnan U,
Winichagoon P, Flores R, Martorell R. Long-term effects of iron and zinc
supplementation during infancy on cognitive function at 9 y of age in
northeast Thai children: A follow-up study. Am J Clin Nutr.
2011;93:636-43.
35. Wasantwisut E, Winichagoon P,
Chitchumroonchokchai C, Yamborisut U, Boonpraderm A, Pongcharoen T,
et al. Iron and zinc supplementation improved iron and zinc status,
but not physical growth, of apparently healthy, breast-fed infants in
rural communities of Northeast Thailand. J Nutr. 2006;136:2405-11.
36. Wieringa FT, Berger J, Dijkhuizen MA, Hidayat A,
Ninh NX, Utomo B, et al. Combined iron and zinc supplementation
in infants improved iron and zinc status, but interactions reduced
efficacy in a multicountry trial in Southeast Asia. J Nutr.
2007;137:466-71.
37. Dirren H, Barclay D, Ramos JG, Lozano R, Montalvo
MM, Davila N, et al. Zinc supplementation and child growth in
Ecuador. Adv Exp Med Biol. 1994;352:215-22.
38. El-Farghali O, El-Wahed MA, Hassan NE, Imam S,
Alian K. Early zinc supplementation and enhanced growth of the low-birth
weight neonate. Open Access Maced J Med Sci. 2015;3:63-8.
39. Elizabeth KE, Sreedevi P, Narayanan SN. Outcome
of nutritional rehabilitation with and without zinc supplementation.
Indian Pedaitr. 2000;37:647-50.
40. Fahmida U, Rumawas JSP, Utomo B, Patmonodewo S,
Schultnik W. Zinc-iron, but not zinc-alone supplementation, increased
linear growth of stunted infants with low haemoglobin. Asia Pacific J
Clin Nutr. 2007;16:301-9.
41. Fischer Walker CL, Baqui AH, Ahmed S, Zaman K, El
Arifeen S, Begum N, et al. Low-dose weekly supplementation of
iron and/or zinc does not affect growth among Bangladeshi infants. Eur J
Clin Nutr. 2010;63:87-92.
42. Gardner JMM, Powell CA, Baker-Henningham H,
Walker SP, Cole TJ, Grantham-McGregor SM. Zinc supplementation and
psychosocial stimulation: Effects on the development of undernourished
Jamaican children. Am J Clin Nutr. 2005;82:399-405.
43. Garenne M, Becher H, Ye Y, Kouyate B, Muller O.
Sex-specific responses to zinc supplementation in Nouna, Burkina Faso. J
Paedaitr Gastroenterol Nutr. 2007;44:619-628.
44. Muller O, Becher H, van Zweeden AB, Ye Y, Diallo
DA, Konate AT, et al. Effect of zinc supplementation on malaria
and other causes of morbidity in west African children: randomised
double blind placebo controlled trial. BMJ. 2001;322:1567.
45. Muller O, Garenne M, Rietmaier P, van Zweeden AB,
Kouyate B, Becher H. Effect of zinc supplementation on growth in West
African children: A randomized double-blind placebo-controlled trial in
rural Burkina Faso. Int J Epidemiol. 2003;32:1098-102.
46. Hershkovitz E, Printzman L, Segev Y, Levy J,
Phillip M. Zinc supplementation increases the level of serum
insulin-like growth factor-i but does not promote growth in infants with
nonorganic failure to thrive. Horm Res. 1999;52:200-204.
47. Abbeddou S, Hess SY, Jimenez EY, Some JW, Vosti
SA, Guissou RM, et al. Comparison of methods to assess adherence
to small-quantity lipid-based nutrient supplements(SQ-LNS) and
dispersible tablets among young Burkinabé children participating in a
community-based intervention trial. Maternal Child Nutr. 2015;11(Suppl
4):90-104.
48. Hess SY, Abbeddou S, Jimenez EY, Some JW, Vosti
SA, Guissou RM, et al. Small-quantity lipid-based nutrient
supplements, regardless of their zinc content, increase growth and
reduce the prevalence of stunting and wasting in young Burkinabe
children: A cluster-randomized trial. PLoS One. 2015;10:e0122242.
49. Prado EL, Abbeddou S, Jimenez EY, Some JW,
Ouedraogo ZP, Vosti SA, et al. Lipid-based nutrient supplements
plus malaria and diarrhea treatment increase infant development scores
in a cluster-randomized trial in Burkina Faso. J Nutr. 2016;146:814-22.
50. Some JW, Abbeddou S, Yakes Jimenez E, Hess SY,
Ouédraogo ZP, Guissou RM, et al. Effect of zinc added to a daily
small-quantity lipid-based nutrient supplement on diarrhoea, malaria,
fever and respiratory infections in young children in rural Burkina
Faso: a cluster randomised trial. BMJ Open. 2015;5:e007828.
51. Hong ZY, Zhang YW, Xu JD, Zhou JD, Gao XL, Liu
XG, et al. Growth promoting effect of zinc supplementation in
infants of high-risk pregnancies. Chin Med J (Engl). 1992;105:844-8.
52. Islam MN, Chowdhury M, Siddika M, Qurishi SB,
Bhuyian MKJ, Hoque MM, et al. Effect of oral zinc supplementation
on the growth of preterm infants. Indian Pediatr. 2010;47:845-49.
53. Lind T, Lönnerdal B, Stenlund H, Gamayanti IL,
Ismail D, Seswandhana R, et al. A community-based randomized
controlled trial of iron and zinc supplementation in Indonesian infants:
effects on growth and development. Am J Clin Nutr. 2004;80:729-36.
54. Ashworth A, Morris SS, Lira PIC,
Grantham-McGregor SM. Zinc supplementation, mental development and
behaviour in low birth weight term infants in northeast Brazil. Eur J
Clin Nutr. 1998;52:223-7.
55. Lira PIC, Ashworth A, Morris SS. Effect of zinc
supplementation on the morbidity, immune function,and growth of
low-birth-weight, full-term infants in northeast Brazil. Am J Clin Nutr.
1998;68:418S-24S.
56. Locks L, Manji K, McDonald C, Kupka R, Kisenge R,
Aboud S, et al. Effect of zinc & multiple micronutrient
supplements on growth in Tanzanian children. Faseb J. 2015;29.
57. Locks LM, Manji KP, McDonald CM, Kupka R, Kisenge
R, Aboud S, et al. Effect of zinc and multivitamin
supplementation on the growth of Tanzanian children aged 6–84 wk: A
randomized, placebo-controlled, double-blind trial. Am J Clin Nutr.
2016;103:910-8.
58. Long KZ, Montoya Y, Hertzmark E, Santos JI,
Rosado JL. A double-blind, randomized, clinical trial of the effect of
vitamin A and zinc supplementation on diarrheal disease and respiratory
tract infections in children in Mexico City, Mexico. Am J Clin Nutr.
2006;83:693-700.
59. Long KZ, Rosado JL, Montoya Y, Solano ML,
Hertzmark E, Dupont HL, et al. Effect of Vitamin A and zinc
supplementation on gastrointestinal parasitic infections among mexican
children. Pediatrics. 2007;120:e846-855.
60. Makonnen B, Venter A, Joubert G. A Randomized
Controlled Study of the Impact of Dietary Zinc Supplementation in the
Management of Children with Protein–Energy Malnutrition in Lesotho. I:
Mortality and Morbidity. J Trop Pediatr. 2003;49:340-52.
61. Makonnen B, Venter A, Joubert G. A randomized
controlled study of the impact of dietary zinc supplementation in the
management of children with protein-energy malnutrition in Lesotho. II:
Special investigations. J Trop Pediatr. 2003;49:353-60.
62. Mathur NB, Agarwal DK. Zinc supplementation in
preterm neonates and neurological development: a randomized controlled
trial. Indian Pediatr. 2015;52:951-55.
63. Meeks Gardner JM, Witter MM, Ramdath DD. Zinc
supplementation: effects on the growth and morbidity of undernourished
Jamaican children. Eur J Clin Nutr. 1998;52:34-39.
64. Mozaraffi-Khosravi H, Shakiba M, Eftekhari M,
Fatehi F. Effects of zinc supplementation on physical growth in
2-5-year-old children. Biol Trac Elem Res. 2009;128:118-27.
65. Olney DK, Kariger PK, Stoltzfus RJ, Khalfan SS,
ALi NS, Tielsch JM, et al. Developmental effects of micronutrient
supplementation and malaria in Zanzibari children. Early Human Dev.
2013;89:667-74.
66. Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali
NS, Tielsch JM, et al. Combined iron and folic acid
supplementation with or without zinc reduces time to walk in gun
assisted among zanzibari infants 5- to 11-mo old. J Nutr.
2006;136:2427-34.
67. Hamadani JD, Fuchs GJ, Osendarp SJM, Khatun F,
Huda SN, Grantham-McGregor. Randomized controlled trial of the effect of
zinc supplementation on the mental development of Bangladeshi infants.
Am J Clin Nutr. 2001;74:381-6.
68. Osendarp S, Santosham M, Black RE, Wahed MA, van
Raaij JMA, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo
of life on growth and morbidity of Bangladeshi infants in urban slums.
Am J Clin Nutr. 2002;76:1401-8.
69. Owusu-Ageyi S, Newton S, Mahama E, Febir LG, Ali
M, Adjei K, et al. Impact of vitamin A with zinc supplementation
on malaria morbidity in Ghana. Nutrition J. 2013;12:131.
70. Penny ME, Marin RM, Duran A, Peerson JM, Lanata
CF, Lönnerdal B, et al. Randomized controlled trial of the effect
of daily supplementation with zinc or multiple micronutrients on the
morbidity, growth, and micronutrient status of young Peruvian children.
Am J Clin Nutr. 2004;79:457-65.
71. Penny ME, Peerson JM, Marin RM, Duran A, Lanata
CF, Lönnerdal B, et al. Randomised community based trial of the
effect of zinc supplementation, with and without other micronutrients,
on the duration of persistent childhood diarrhea in Lima, Peru. J
Pediatr. 1999;135:208-17.
72. Radhakrishna KV, Hemalatha R, Geddam JJB, Kumar
PA, Balakrishna N, Shatrugna V. Effectiveness of zinc
supplementation to full term normal infants: A community based double
blind, randomized, controlled, clinical trial. PLoS One. 2013;8:e61486.
73. Rahman MM, Tofail F, Wahed MA, Fuchs GJ, Baqui
AH, Alvarez JO. Short-term supplementation with zinc and vitamin A has
no significant effect on the growth of undernourished Bangladeshi
children. Am J Clin Nutr. 2002;75:87-91.
74. Ram Kumar TV, Ramji S. Effect of zinc
supplementation on growth in very low birth weight infants. J Trop
Paediatr. 2012;58:50-54.
75. Bentley ME, Caulfield LE, Ram M, Santizo MC,
Hurtado E, Rivera JA, et al. Zinc supplementation affects the
activity patterns of rural Guatemalan infants. J Nutr. 1997;127:1333-8.
76. Rivera JA, Ruel MT, Santizo MC,Lo¨ nnerdal B,
Brown KH. Zinc supplementation improves the growth of stunted rural
Guatemalan infants. J Nutr. 1998;128:556-62.
77. Ruel MT, Rivera JA, Santizo M, Lo¨nnerdal B,
Brown KH. Impact of zinc supplementation on morbidity from diarrhea and
respiratory infections among rural Guatemalan children. Pediatrics.
1997;99:808-13.
78. Allen LH, Rosado JL, Casterline JE, López P,
Muñoz E, Garcia OP, et al. Lack of hemoglobin response to iron
supplementation in anemic Mexican preschoolers with multiple
micronutrient deficiencies. Am J Clin Nutr. 2000;71:1485-94.
79. Rosado JL, Lopez P, Mufioz E, Martinez H, Allen
LH. Zinc supplementation reduced morbidity, but neither zinc nor iron
supplementation affected growth or body composition of Mexican
preschoolers. Am J Clin Nutr. 1997;65:13-9.
80. Rosado JL. Separate and joint effects of
micronutrient deficiencies on linear growth. J Nutr. 1999;129:531S-3S.
81. Rosado JL, Caaman˜o MC, Montoya YA, de Lourdes
Solano M, Santos JI, Long KZ. Interaction of zinc or vitamin A
supplementation and specific parasite infections on Mexican infants’
growth: a randomized clinical trial. Eur J Clin Nutr. 2009;63:1176-84.
82. Ruz M, Castillo-Duran C, Lara X, Codoceo J,
Rebolledo A, Atalah E. A 14-mo zinc-supplementation trial in apparently
healthy Chilean preschool children. Am J Clin Nutr. 1997;66:1406-13.
83. Sampaio DLB, Mattos APD, Rebeiro TCM, Liete ME,
Cole CR, Costa-Ribeiro Jr H. [Zinc and other micronutrients
supplementation through the use of sprinkles: impact on the occurrence
of diarrhea and respiratory infections in institutionalized children]
Article in Spanish. J Pediatr. (Rio J) 2013;89:286-93.
84. Sanchez J, Villada OA, Rojas ML, Montaya L, Diaz
A, Vargas C, et al. [Effect of zinc amino acid chelate and zinc
sulfate in the incidence of respiratory infection and diarrhea among
preschool children in child daycare centers]. Article in Spanish.
Biomédica. 2014;34:79-91.
85. Sempértegui F, Estrella B, Correa E, Aguirre L,
Saa B, Torres M, et al. Effects of short-term zinc
supplementation on cellular immunity, respiratory symptoms, and growth
of malnourished Equadorian children. Eur J Clin Nutr. 1996;50:42-6.
86. Shankar AH, Geston B, Baisor M, Paino J, Tamija
S, Adiguma T, et al. The influence of zinc supplementation on
morbidity due to Plasmodium falciparum: A randomized trial in preschool
children in Papua New Guinea. Am J Trop Med Hyg. 2000;62:663-9.
87. Shrivastava SP, Roy AK, Jana UK. Zinc
supplementation in protein energy malnutrition. Indian Pediatr.
1993;30:779-82.
88. Silva APR, Vitolo MR, Zara LF, Castro CF.
[Effects of zinc supplementation on 1- to 5-year old children]. Article
in Spanish. J Pediatr. (Rio J) 2006;82:227-31.
89. No Authors Listed. Effect of provision of
micronutrient supplementation on growth and morbidity among young
children in Pakistan. Arch Dis Child. 2013;98:691.
90. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J,
Ahmed I, et al. Effect of provision of daily zinc and iron with
several micronutrients on growth and morbidity among young children in
Pakistan: a cluster-randomised trial. Lancet. 2013;382:29-40.
91. Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B,
Rajendran K, et al. Impact of zinc supplementation on diarrheal
morbidity and growth pattern of low birth weight infants in Kolkata,
India: A randomized, double-blind, placebo-controlled, community-based
study. Pediatrics. 2003;112:1327-32.
92. Siegel EH, Kordas K, Stoltzfus RJ, Katz J, Khatry
SK, LeClerq SC, et al. Inconsistent effects of iron-folic acid
and/or zinc supplementation on the cognitive development of infants. J
Health Popul Nutr. 2011;29:593-604.
93. Surkan PJ, Shankar M, Katz J, Siegel EH, LeClerq
SC, Khatry SK, et al. Beneficial effects of zinc supplementation
on head circumference of Nepalese infants and toddlers: a randomized
controlled trial. Eur J Clin Nutr. 2012;66: 836-42.
94. Taneja S, Bhandari N, Rongsen-Chandola T,
Mahalanabis D, Fontaine O, Bhan MK. Effect of zinc supplementation on
morbidity and growth in hospital-born, low-birth-weight infants. Am J
Clin Nutr. 2009;90:385-91.
95. Taneja S, Strand TA, Sommerfelt H, Bahl R,
Bhandari N. Zinc supplementation for four months does not affect growth
in young North Indian children. J Nutr. 2010;140:630-4.
96. Umeta M, West CE, Haidar J, Deurenberg P,
Hautvast J. Zinc supplementation and stunted infants in Ethiopia: A
randomised controlled trial. Lancet. 2000;255:2121-6.
97. Vasudevan A, Shedurnikar N, Kotecha PV. Zinc
supplementation in severe malnutrition. Indian Pediatr. 1997;34:236-8.
98. Wuehler SE, Sempértegui F, Brown KH.
Dose-response trial of prophylactic zinc supplements, with or without
copper, in young Ecuadorian children at risk of zinc deficiency. Am J
Clin Nutr. 2008;87:723-33.
99. Yang YX, Han JH, Shao XP, He M, Bian LH, Wang Z,
et al. Effect of micronutrient supplementation on the growth of
preschool children in China. Biomed Environ Sci. 2002;15:196-202.
100. Zlotkin S, Arthur P, Schauer C, Antwi KY, Yeung
G, Piekarz A. Home-fortification with iron and zinc sprinkles or iron
sprinkles alone successfully treats anemia in infants and young
children. J Nutr. 2003;133:1075-80.
101. Imdad A, Bhutta ZA. Effect of preventive zinc
supplementation on linear growth in children under 5 years of age in
developing countries: A meta-analysis of studies for input to the lives
saved tool. BMC Public Health. 2011;11:S22.
102. Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan
XHS, Chan ES, et al. Zinc supplementation for preventing
mortality, morbidity, and growth failure in children aged 6 months to 12
years of age. Cochrane Database Syst Rev. 2014;5:CD009384.
103. Liu E, Pimpin L, Shulkin M, Kranz S, Duggan CP, Mozaffarian D,
et al. Effect of zinc supplementation on growth outcomes in
children under 5 years of age. Nutrients. 2018;10: pii: E377. doi:
10.3390/nu10030377.

