|
|
|
Indian Pediatr 2021;58: 219-223 |
 |
Evolution Bites - Timeworn Inefficacious Snakebite Therapy in
the Era of Recombinant Vaccines
|
|
Navneet Kaur, Ashwin Iyer and Kartik Sunagar
From Evolutionary Venomics Lab, Centre for Ecological Sciences,
Indian Institute of Science. Bangalore, Karnataka, India.
Correspondence to: Dr Kartik Sunagar, Evolutionary Venomics Lab,
Centre for Ecological Sciences, Indian Institute of Science. Bengaluru
560 012, Karnataka, India.
Email: [email protected]
|
|
Snakebite is a neglected tropical disease that inflicts severe
socioeconomic burden on developing countries by primarily affecting
their rural agrarian populations. India is a major snakebite hotspot in
the world, as it accounts for more than 58,000 annual snakebite
mortalities and over three times that number of morbidities. The only
available treatment for snakebite is a commercially marketed polyvalent
antivenom, which is manufactured exclusively against the ‘big four’
Indian snakes. In this review, we highlight the influence of ecology and
evolution in driving inter- and intra-specific venom variations in
snakes. We describe the repercussions of this molecular variation on the
effectiveness of the current generation Indian antivenoms in mitigating
snakebite pathologies. We highlight the disturbing deficiencies of the
conventional animal-derived antivenoms, and review next-generation
recombinant antivenoms and other promising therapies for the efficacious
treatment of this disease.
Keywords: Antivenom, Evolution, Proteomics,
Venom.
|
|
V
enom is a complex biochemical concoction
that is contrasted from poisons in being
actively injected by the producing animal into
the target prey or predator. Given their medical importance, snake
venoms have fascinated humans since time immemorial, and have been
extensively studied to date. Animal venoms can be chemically constituted
by proteins, amino acids, carbohydrates, salts, and polyamines [1].
Snake venoms, however, are primarily proteinaceous. Historically, an
anthropocentric bias has led to an erroneous understanding that only
animals capable of inflicting medically significant envenomation are
‘venomous’. However, venoms represent an evolutionary adaptation for
self-defense and prey capture. Therefore, venom may attain remarkable
specificity towards target animals and become ineffective against
non-target species. For instance, certain venom toxins in arboreal ‘tree
snakes’ (e.g., genus Boiga) exhibit extreme potency towards their
avian and reptilian prey, while exhibiting reduced effectiveness against
mammals, including humans. The potency and composition of snake venom
cocktails are driven by diverse factors, such as varying diet (e.g.,
ontogenetic dietary shifts), geographical distribution and environmental
conditions [2,3].
A MILLION DEATHS
Despite being the non-target species, accidental
snake envenoming in humans has resulted in hundreds of thousands of
deaths and disabilities worldwide. Snake envenoming affects between 4.5
to 5.4 million people globally, inflicting over 100,000 deaths and
400,000 disabilities, annually [4]. Tragically, India accounts for
58,000 snakebite deaths and three-times as many immutable morbidities,
making it a major snakebite hotspot [5]. Snakebite disproportionately
affects the impoverished rural populations that often lack essential
health infrastructure. As most bite victims are the primary breadwinners
of their families, snakebite devastates far greater numbers of lives and
livelihoods than currently recognized. Although snakebites kill nearly
as many people in India as HIV infections, they only receive a fraction
of the research attention and medical investment devoted to HIV. Since
snakebite primarily affects the poor, and young males are at the highest
risk of getting bitten, it results in severe socioeconomic consequences
in developing countries. These considerations have led to the enlisting
of snake envenoming as a high priority ‘neglected tropical disease’
(NTD) by the World Health Organization (WHO) [4].
VENOMS TO DRUGS
On the flip side, snake venoms have saved many more
lives than they have taken. Millions of years of evolution has resulted
in diverse snake venom toxins with remarkable target specificities, and
this property is being extensively exploited for drug discovery. Many
snake venom proteins have been engineered into highly specific and
efficient lifesaving drugs. For instance, Captopril, an
angiotensin-converting enzyme inhibitor used for the treatment of
hypertension, is derived from the venom of the Brazilian pit viper,
Bothrops jararaca, and has become the poster child for drug
discovery from snake venoms. This exceptional drug has saved millions of
lives globally since its introduction in the early 1980s. Many other
snake venom-derived therapeutics for the treatment of various diseases,
including multiple sclerosis, thrombosis, and cardiovascular diseases,
are under various phases of clinical trials [6].
CLINICAL CONSEQUENCES OF VENOM VARIATION
Antivenom is the mainstay treatment of snakebite,
whose manufacturing protocols have remained essentially unchanged since
their inception in the late 1800s: purification of immunoglobulins (IgG)
from horses hyperimmunized with sublethal and subtoxic doses of snake
venom (Fig. 1). The efficacy of conventional antivenom is
restricted to the immunogenic potential of venoms used in its
manufacture. Since venom is an adaptive trait that underpins various
quotidian functions, it often exhibits dramatic inter-(between) and
intra-specific (within species) variability. This variation may result
in very distinct clinical outcomes and, thus, severely limits the
cross-population/species antivenom efficacy – i.e., treatment of
snakebites of one population/species using antivenom raised for another.
However, for the commercial production of Indian antivenoms, venoms are
exclusively sourced in Tamil Nadu from the so-called ‘big four’ snakes:
the spectacled cobra (Naja naja), common krait (Bungarus
caeruleus), Russell’s viper (Daboia russelii), and saw-scaled
viper (Echis carinatus). Moreover, India is abode to many other
medically important snake species capable of inflicting fatalities and
morbidities in their accidental human bite victims. Northeast India, for
example, is devoid of the ‘big four’ snakes, but is dominated by other
medically important snake species. Unfortunately, however, a single
polyvalent antivenom manufactured for treating bites from the ‘big four’
snakes is marketed throughout the country, including in regions that
lack these species. As Indian antivenoms fail to account for the inter-
and intra-specific variability in venoms, they are preclinically shown
to be less effective in mitigating bites from the pan-Indian populations
of both ‘big four’ snakes and the ‘neglected many’, medically important
snakes for which antivenoms are not manufactured [7,8].
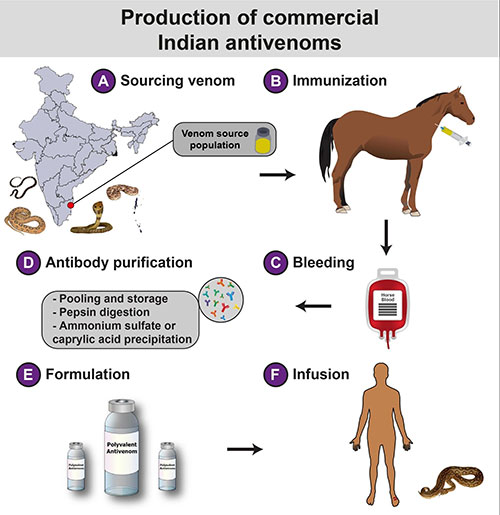 |
|
The manufacturing process of Indian
antivenoms involves a) sourcing of venoms from the ‘big four’
snakes in a couple of districts in Tamil Nadu, followed by b)
the immunization of healthy equines with these venoms in
sublethal and subtoxic doses; c) immunized equines are then bled
and the plasma is separated from the blood. The processed blood
without plasma is mixed with saline and often reintroduced into
the immunized animal; d) The serum is first digested with pepsin
to cleave off immunoglobulin heavy chains, resulting in divalent
F(ab’)2 fragments, followed by the treatment with ammonium
sulfate or caprylic acid to precipitate antibodies, and
ultracentrifugation of the precipitate to obtain purified
antibodies; e) The purified antivenom is formulated either in
liquid or lyophilized form before being marketed for f) the
treatment of snakebite victims.
Fig. 1 The production of conventional
Indian antivenoms.
|
DISTURBING DEFICIENCIES OF ANTIVENOM
Hyperimmunization of animals with crude ‘whole’
venoms, which often contain antigens and other impurities, is the major
shortcoming of the conventional antiserum therapy, as it increases the
amount of contaminant antibodies in the finished product. In fact, the
proportion of therapeutically relevant antibodies in an antivenom vial
may be lower than 10-15% of the content [9], necessitating the
requirement of a considerably large number of vials for efficacious
treatment. This, in turn, increases the cost of treatment to the point
that it becomes unaffordable to many in low- and middle-income
countries. Fortunately, Indian antivenoms are heavily subsidized by the
government and are freely administered without charges in government
hospitals. Infusion of substantial amounts of therapeutically redundant
antivenom; however, leads to complications, including serum sickness and
the fatal anaphylactic shock. It is, therefore, not surprising that
nearly 80% of snakebite victims who were treated with the Indian
antivenoms were found to exhibit multiple adverse effects [10]. This
highlights the pressing need to increase the dose effectiveness of
currently available commercial antivenoms in the country.
In addition to the low dose efficacy, the poor
cross-species/population neutralization capability are the other major
deficiencies of the commercial Indian antivenoms. The marketed
antivenoms, which are manufactured exclusively against the ‘big four’
snake venoms from a couple of districts in Tamil Nadu, have been shown
to poorly mitigate the toxic effects inflicted by the geographically
disparate populations of the ‘big four’ snakes and the ‘neglected many’
[7,8]. Unfortunately, the effectiveness of commercial Indian antivenoms
has been largely evaluated by in vitro methods [e.g., enzyme-linked
immunosorbent assay (ELISA), western blotting and immunochromatography (Web
Table I)]. In contrast to in vivo experiments in the mouse model
(e.g., Effective Dose 50 or ED 50),
in vitro methods do not reveal the underlying neutralization potencies
of antivenoms, but are only useful for understanding their venom
recognition potential. Furthermore, low-molecular-weight toxins, such as
three-finger toxins (3FTx), which dominate the venoms of many elapid
snakes (e.g., Naja and Bungarus spp.) and are responsible for the morbid
and fatal symptoms, exhibit poor immunogenicity, likely leading to a
reduced proportion of neutralizing antibodies against them [22].
UPCOMING THERAPIES FOR SNAKEBITES
To date, hyperimmunized animal-derived antivenom
remains the only available treatment for snakebites. The inefficacy of
such antivenoms in neutralizing the toxic effects of distinct medically
important species and their geographically disparate populations have
been well-documented. In recent times, several innovative strategies are
being explored to develop next-generation antivenoms with increased
potency, paraspecificity, and cost-effectiveness. Some of these
strategies have been briefly described below.
Phage display: It facilitates the identification
of antibodies specific to toxins of interest. Phage display essentially
involves biopanning of antibody phage display libraries against a
particular antigen, in this case, venom proteins, followed by the
amplification and enrichment of the antigen-specific library. Selected
phages are used for infecting bacteria, which are then allowed to
express toxin-specific antibody fragments. Specific antibodies against
various toxin types can also be combined to form a biosynthetic
oligoclonal antibody (BOA) cocktail, which exhibits less batch-to-batch
variation and increased efficacy and safety profiles than the
conventional antivenoms [23]. Phage display technology has been shown to
be effective in characterizing antibodies against medically important
snake venom toxins, including crotoxin, cobratoxin, and dendrotoxin
[24].
Synthetic epitope strategy: Next-generation
antivenoms containing toxin-specific antibodies could also be produced
through novel immunization strategies, such as immunization with
synthetic epitope-strings. Herein, strings of nucleotide sequences
coding for specific regions of various toxins are cloned into expression
vectors and injected into animals for eliciting toxin-specific antibody
response [25].
Aptamers: The use of aptamers, oligonucleotides
or oligopeptides that bind to target molecules with high specificity,
have also been considered for the development of novel antivenom
therapies [26]. This strategy can be advantageous over animal-derived
antibodies in terms of production, affordability, and ethical
considerations.
Mimotopes: Structurally mimicking regions of
clinically important toxins known as ‘mimotopes’ can elicit immune
responses and generate toxin-specific antibodies. Examples include
mutalysin-II mimotopes that have been shown to neutralize hemorrhagic
activity induced by Lachesis muta venom [27]. These mimotopes are
usually identified from a phage display library and have high
specificity and stability.
Nanoparticle engineering: Another alternative to
the current intravenous antivenom administration is the subcutaneous use
of nanoparticle drug delivery systems that can facilitate the controlled
release of highly stable toxin neutralizing nanoparticles. Synthetic
hydrogel nanoparticles, for example, have been shown to inhibit
phospholipase A 2 (PLA2)
and 3FTx pathologies [28,29]. Similarly, nanoparticles, such as C60
fullerene, have been shown to exhibit significant neutralization against
rattlesnake envenomation [30].
In addition, several small molecular inhibitors, such
as varespladib are currently being tested for their ability to
neutralize snakebite pathologies [28]. Unfortunatel, very few products
originating from these next-generation technologies are under various
phases of clinical trials, while most others are being preclinically
evaluated. Thus, while the aforementioned technologies are promising and
are likely to result in highly efficacious and affordable snakebite
treatment therapies, they are far from fruition. It is therefore
imperative, in the interim, to address the deficiencies of the current
generation Indian antivenoms. Procurement of venoms from the pan-Indian
populations of ‘big four’ and other medically important snakes by region
for the production of region-specific antivenoms, while also accounting
for the ecological specializations and molecular evolutionary dynamics
of venoms of clinically relevant species, could be effective in
countering the geographic and phyletic variations in venom compositions
and potencies. Furthermore, adoption of novel immunization strategies
(e.g., the use of medically important toxin fractions and/or poor
immunogenic toxin proteins for animal immunization) and purification
technologies (e.g., chromatographic purification of antivenoms during
manufacture) are highly likely to increase the proportion of
therapeutically significant antibodies in the marketed product. Thus, in
the absence of next-generation antivenoms, these measures are
anticipated to save the lives, limbs and livelihoods of India’s hundred
thousand annual snakebite victims.
Acknowledgements: RR Senji Laxme and Suyog
Khochare (Evolutionary Venomics Lab, IISc) for their inputs.
Contributors: All authors contributed equally to
the manuscript.
Funding: KS: Department of Science and Technology
(DST) INSPIRE Faculty Award, DST-FIST, DBT-IISc Partnership Program, and
the DBT/Wellcome Trust India Alliance Fellowship.
Competing interest: None stated.
REFERENCES
1. Sunagar K, Casewell N, Varma S, Kolla R, Antunes
A, Moran Y. Deadly innovations: Unraveling the molecular evolution of
animal venoms. Venom Genomics and Proteomics; Springer. 2014.p.1-23.
2. Suranse V, Iyer A, Jackson T, Sunagar K. Early
origin and diversification of the enigmatic reptilian venom cocktail.
Systematic Association Special Volume. 2020.
3. Casewell NR, Jackson TN, Laustsen AH, Sunagar K.
Causes and consequences of snake venom variation. Trends in
pharmacological sciences. 2020.
4. Gutierrez JM, Calvete JJ, Habib AG, Harrison RA,
Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers.
2017;3:17063.
5. Suraweera W, Warrell D, Whitaker R, Menon GR,
Rodrigues R, Fu SH, Begum R, Sati P, Piyasena K, Bhatia M, Brown P.
Trends in snakebite mortality in India from 2000 to 2019 in a nationally
representative mortality study. medRxiv. 2020 Jan 1.
6. Mohamed Abd El-Aziz T, Soares AG, Stockand JD.
Snake venoms in drug discovery: valuable therapeutic tools for life
saving. Toxins (Basel). 2019;11:564.
7. Laxme RS, Khochare S, de Souza HF, et al. Beyond
the ‘big four’: Venom profiling of the medically important yet neglected
Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl Trop
Dis. 2019;13:12.
8. Shashidharamurthy R, Kemparaju K. Region-specific
neutralization of Indian cobra (Naja naja) venom by polyclonal
antibody raised against the eastern regional venom: A comparative study
of the venoms from three different geographical distributions. Int
Immunopharmacol. 2007;7:61-9.
9. Casewell NR, Cook DA, Wagstaff SC, et al.
Pre-clinical assays predict pan-African Echis viper efficacy for
a species-specific antivenom. PLoS Negl Trop Dis. 2010;4:e851.
10. Ariaratnam CA, Sjostrom L, Raziek Z, et al. An
open, randomized comparative trial of two antivenoms for the treatment
of envenoming by Sri Lankan Russell’s viper (Daboia russelii russelii).
Trans R Soc Trop Med Hyg. 2001;95:74-80.
11. Mukherjee AK, Maity CR. Biochemical composition,
lethality and pathophysiology of venom from two cobras – Naja naja
and N. kaouthia. Comp Biochem Physiol B Biochem Mol Biol.
2002;131:125-32.
12. Shashidharamurthy R, Jagadeesha DK, Girish KS,
Kemparaju K. Variations in biochemical and pharmacological properties of
Indian cobra (Naja naja naja) venom due to geographical
distribution. Mol Cell Biochem. 2002;229:93-101.
13. Kadali R, Kadiyala G, Gurunathan J. Pre clinical
assessment of the effectiveness of modified polyvalent antivenom in the
neutralization of Naja naja venom toxicity. Biotechnol Appl Bioc.
2016;63:827-33.
14. Chanda A, Kalita B, Patra A, Senevirathne WDST,
Mukherjee AK. Proteomic analysis and antivenomics study of Western India
Naja naja venom: correlation between venom composition and
clinical manifestations of cobra bite in this region. Expert Rev
Proteomics. 2019;16:171-84.
15. Prasad NB, Uma B, Bhatt SK, Gowda VT. Comparative
characterisation of Russell’s viper (Daboia/Vipera russelli)
venoms from different regions of the Indian peninsula. Biochim Biophys
Acta. 1999;1428:121-36.
16. Sharma M, Gogoi N, Dhananjaya B, Menon JC, Doley
R. Geographical variation of Indian Russell’s viper venom and
neutralization of its coagulopathy by polyvalent antivenom. Toxin
Reviews. 2014;33:7-15.
17. Kalita B, Singh S, Patra A, Mukherjee AK.
Quantitative proteomic analysis and antivenom study revealing that
neurotoxic phospholipase A2 enzymes, the major toxin class of Russell’s
viper venom from southern India, shows the least immuno-recognition and
neutralization by commercial polyvalent antivenom. Int J Biol Macromol.
2018;118:375-85.
18. Pla D, Sanz L, Quesada-Bernat S, et al.
Phylovenomics of Daboia russelii across the Indian subcontinent.
Bioactivities and comparative in vivo neutralization and in
vitro third-generation antivenomics of antivenoms against venoms
from India, Bangladesh and Sri Lanka. J Proteom. 2019;207:103443.
19. Patra A, Kalita B, Chanda A, Mukherjee AK.
Proteomics and antivenomics of Echis carinatus carinatus venom:
Correlation with pharmacological properties and pathophysiology of
envenomation. Sci Rep. 2017;7:1-17.
20. Patra A, Chanda A, Mukherjee AK. Quantitative
proteomic analysis of venom from Southern India common krait (Bungarus
caeruleus) and identification of poorly immunogenic toxins by
immune-profiling against commercial antivenom. Expert Rev Proteomics.
2019;16:457-69.
21. Sunagar K, Khochare S, Laxme RS, et al. A wolf in
another wolf’s clothing: Post-genomic regulation dictates venom profiles
of medically-important cryptic kraits in India [pre-print]. bioRxiv. 2020.12.15.422536
22. Fernández J, Alape-Girón A, Angulo Y, et al.
Venomic and antivenomic analyses of the Central American coral snake,
Micrurus nigrocinctus (Elapidae). J Proteome Res. 2011;10:1816-27.
23. Kini RM, Sidhu SS, Laustsen AH. Biosynthetic
oligoclonal antivenom (boa) for snakebite and next-generation treatments
for snakebite victims. Toxins. 2018;10:534.
24. Kulkeaw K, Sakolvaree Y, Srimanote P, et al.
Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia,
neurotoxin. J Proteomics. 2009;72:270-82.
25. Ferreira RN, Machado de Avila RA, Sanchez EF, et
al. Antibodies against synthetic epitopes inhibit the enzymatic activity
of mutalysin II, a metalloproteinase from bushmaster snake venom.
Toxicon. 2006;48:1098-103.
26. Ye F, Zheng Y, Wang X, et al. Recognition of
Bungarus multicinctus venom by a DNA aptamer against beta-bungarotoxin.
PLoS One. 2014;9:e105404.
27. Machado de Avila RA, Stransky S, Velloso M, et
al. Mimotopes of mutalysin-II from Lachesis muta snake venom
induce hemorrhage inhibitory antibodies upon vaccination of rabbits.
Peptides. 2011;32:1640-6.
28. Lewin M, Samuel S, Merkel J, Bickler P.
Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor
of snake venom phospholipase A2 and a possible pre-referral treatment
for envenomation. Toxins. 2016;8:248.
29. O’Brien J, Lee SH, Gutierrez JM, Shea KJ.
Engineered nanoparticles bind elapid snake venom toxins and inhibit
venom-induced dermonecrosis. PLoS Negl Trop Dis. 2018;12:e0006736.
30. Karain BD, Lee MKH, Hayes WK. C60 Fullerenes as a novel treatment
for poisoning and envenomation: A proof-of-concept study for snakebite.
J Nanosci Nanotech. 2016;16:7764-71.
|
|
|
 |
|

