|
|
|
Indian Pediatr 2016;53: 250 -252 |
 |
Worsening of Callus Hyperplasia after
Bisphosphonate Treatment in Type V Osteogenesis Imperfecta
|
|
Prajnya Ranganath, #Joshi
Stephen, *Raju Iyengar and
#Shubha R Phadke
From the Departments of Medical Genetics and *Orthopaedics, Nizam’s
Institute of Medical Sciences, Hyderabad, Telangana; and #Department of
Medical Genetics, SGPGIMS, Lucknow, Uttar Pradesh; India.
Correspondence to: Dr Prajnya Ranganath, Department of Medical
Genetics, Nizam’s Institute of Medical Sciences, Punjagutta, Hyderabad,
Telangana 500 082, India.
Email: [email protected]
Received: June 23, 2015;
Initial review: September 13, 2015;
Accepted: October 31, 2015.
|
|
Background: Type V osteogenesis imperfecta is characterized by
hyperplastic callus formation and interosseus membrane calcification.
Case characteristics: A 16-year-old boy who presented with history
of recurrent fractures, had hard persistent swellings at fracture sites,
and had radiographic features of hyperplastic callus and interosseus
membrane calcification. Outcome: Sequence analysis of the
IFITM5 gene revealed the c.-14 C>T mutation. The patient had
significant exacerbation of callus hyperplasia after initiation of
bisphosphonate therapy, which reversed following cessation of the
treatment. Message: Bisphosphonates may exacerbate callus
hyperplasia, and may therefore have to be used with caution in patients
with type V osteogenesis imperfecta.
Keywords: Bisphosphonates, Fractures,
Osteogenesis imperfecta.
|
|
Osteogenesis imperfecta (OI) is a clinically and
genetically heterogeneous group of disorders characterized by bone
fragility and increased susceptibility to fractures. Around 15 different
types are known, with significant phenotypic overlap amongst the
different types making clinical differentiation difficult [1,2]. Type V
OI has distinct clinical and radiological features, and is associated
with a specific mutation in the IFITM5 (Interferon-induced
transmembrane protein 5; OMIM *614757) gene, which make it relatively
easy to diagnose [3,4].
While bisphosphonate therapy is the standard of care
for most forms of OI, literature pertaining to its use in Type V OI is
limited [5,6]. We report a patient of type V OI, who had exacerbation of
callus hyperplasia on treatment with bisphosphonates.
Case Report
A 16-year-old boy, the first offspring of
non-consanguineous parents, presented with a history of recurrent (five)
fractures following minimal trauma since three years of age, and hard
persistent non-tender swellings at the fracture sites. There was no
history of hearing loss. There were no symptoms suggestive of any other
chronic systemic disease. His developmental milestones and cognitive
functions were normal. There was no significant family history.
The anthropometric measurements were as follows:
height 154 cm (3 rd centile
for age), weight 40 kgs (5th
centile for age) and head circumference 51 cm. Clinical examination
revealed a diffuse, ill-defined, hard swelling over the upper lateral
aspect of the left thigh. There was no other obvious bone deformity. The
sclerae were white and the teeth were normal. Hearing assessment was
normal in both the ears. The patient had limitation in the range of
pronation and supination in both forearms, with a greater degree of
impairment in the right forearm. Systemic examination was normal. Both
parents were normal on clinical evaluation.
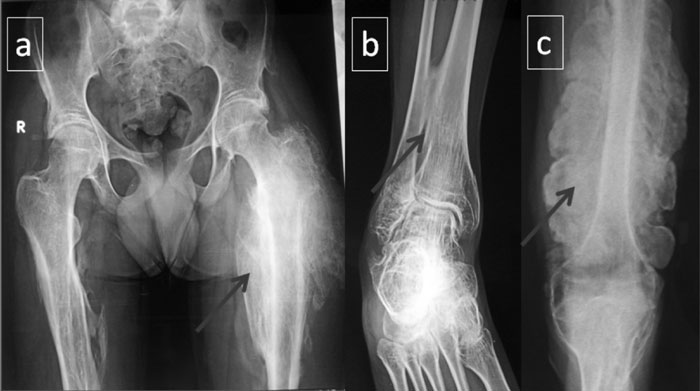
Skeletal radiographs revealed hyperplastic callus in
the upper lateral part of the left femur (extending from the greater
trochanter to the upper thirds of the femur) (Fig. 1a),
radiodense metaphyseal bands in the distal femora, proximal tibias and
distal radii, and compression of the lower thoracic vertebral bodies.
Interosseous membrane calcification was present in both upper and lower
limbs (Fig. 1b). Bone mineral density measured through
dual-energy X-ray absorptiometry (DEXA) at the lumbar spine and femoral
necks bilaterally was found to be low (T score ranging from -3.5 to
-3.9).
 |
|
Fig. 1 (a) Hyperplastic callus
seen in the proximal part of the left femur before the
initiation of bisphosphonate therapy; (b) calcification of the
interosseus membrane seen between the distal parts of the tibia
and fibula; (c) extensive callus formation along the left femur
following initiation of bisphosphonate therapy in the patient.
|
Type V OI was suspected based on the clinical and
radiological features. Sequence analysis of the first exon and the
flanking 5 ˘-untranslated
region of the IFITM5 gene revealed the c.-14C>T mutation, thereby
confirming the diagnosis.
The patient was started on bisphosphonate therapy
(oral alendronate 1 mg/kg/week) with calcium supplementation and was
followed up on a monthly basis. Over the next 10 months, he did not
develop any fractures but there was a progressive increase in callus
formation with extension of the callus from the proximal to the distal
end of the left femur on both the medial and lateral aspects (Fig.
1c), and new callus formation on the right femur and upper and lower
parts of the left tibia and left fibula. As this exacerbation of callus
formation appeared to be chronologically related to the bisphosphonate
therapy, oral alendronate was discontinued. Following cessation of
bisphosphonates, the patient has been on follow-up for three months,
during which time there has been no further increase in the callus
formation, and slight resolution of the callus around the left femur.
Discussion
Type V OI, first described by Glorieux, et al.
[3], is a distinct entity characterized radiologically by calcification
of the interosseous membrane and hyperplastic callus formation, and
histopathologically by a mesh-like appearance of the bone lamellae [3].
It is an autosomal dominant disorder and majority of cases occur
sporadically due to a de novo mutation. As per the recently
proposed nomenclature for OIs, type V OI is now referred to as OI with
calcification in interosseous membranes [1]. The c.-14C>T mutation,
which is till date the only mutation reported to cause type V OI, occurs
in the 5 ˘-untranslated
region of the IFITM5 gene, 14 bp upstream of the annotated
translation initiation codon [4].
As for other forms of OI, management of type V OI
involves supportive therapy to minimize fractures and maximize function,
and orthopaedic intervention for fractures and spinal
compression/deformity. Bisphosphonates (intravenous pamidronate and
zolendronate, and oral alendronate and risedronate), which act by
decreasing bone resorption, are being used for almost two decades in the
management of all forms of OI [7]. There is limited information
regarding the effects of the therapy on callus hyperplasia, which is an
integral component of Type V OI. In a study by Cheung, et al. [5]
in 23 patients with type V OI, pamidronate therapy was not found to
influence the course of hyperplastic callus formation. In another study
of 11 patients with type V OI, the response to pamidronate treatment was
found to be the same as in other types of OI [6]. In our patient, the
exacerbation in callus formation appeared to be chronologically related
to, and thus attributable to the initiation of bisphosphonate therapy.
Treatment with bisphosphonates in experimental models of osteoporosis
has been found to be associated with increased callus size and
mineralization and reduced callus remodelling [8]. Pathogenesis of type
V OI is different from that of the other OI types in that the mutant
allele appears to have a differential tissue-specific and
chronology-specific expression (as suggested by the contradictory
components of osteopenia versus ectopic calcification and
hyperplastic callus), and this may lead to a deviant response to
bisphosphonate therapy in some cases with this OI type [9].
At present, no drug has been found to be beneficial
in reducing callus hyperplasia or interosseous membrane calcification in
Type V OI. Thus, although type V OI is relatively easy to diagnose, it
remains a difficult condition to treat.
Contributors: PR: Clinical evaluation,
diagnosis and management of patient, review of literature, preparation
of manuscript; JS: Molecular genetic analysis of patient, inputs for
manuscript preparation; RI: Clinical evaluation of patient, inputs for
manuscript preparation; SRP: Genetic evaluation of patient, preparation
and review of manuscript.
Funding: Molecular genetic testing was
done through the ICMR-funded project of Dr Shubha Phadke - ICMR -
63/8/2010-BMS. Competing Interests: None stated.
References
1. Van Dijk FS, Sillence DO. Osteogenesis imperfecta:
clinical diagnosis, nomenclature and severity assessment. Am J Med Genet
A. 2014;164A:1470-81.
2. Valadares ER, Carneiro TB, Santos PM, Oliveira AC,
Zabel B. What is new in genetics and osteogenesis imper-fecta
classification? J Pediatr (Rio J). 2014;90:536-41.
3. Glorieux FH, Rauch F, Plotkin H, Ward L, Travers
R, Roughley P, et al. Type V osteogenesis imperfecta: a new form
of brittle bone disease. J Bone Miner Res. 2000;15:1650-8.
4. Takagi M, Sato S, Hara K, Tani C, Miyazaki O,
Nishimura G, et al. A recurrent mutation in the 5'-UTR of IFITM5
causes osteogenesis imperfecta type V. Am J Med Genet A.
2013;161A:1980-2.
5. Cheung MS, Glorieux FH, Rauch F. Natural history
of hyperplastic callus formation in osteogenesis imperfecta type V. J
Bone Miner Res. 2007;22:1181-6.
6. Zietlin L, Rauch F, Travers R, Munns C, Glorieux
FH. The effect of cyclical intravenous pamidronate in children and
adolescents with osteogenesis imperfecta type V. Bone. 2006;38:13-20.
7. Dwan K, Phillipi CA, Steiner RD, Basel D.
Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database
Syst Rev. 2014;7:CD005088.
8. Goldhahn J, Féron JM, Kanis J, Papapoulos S,
Reginster JY, Rizzoli R, et al. Implications for fracture healing
of current and new osteoporosis treatments: An ESCEO consensus paper.
Calcif Tissue Int. 2012;90:343-53.
9. Cho TJ, Lee KE, Lee SK, Song SJ, Kim KJ, Jeon D,
et al. A single recurrent mutation in the 52 -UTR of IFITM5
causes osteogenesis imperfecta type V. Am J Hum Genet. 2012;91:343-8.
|
|
|
 |
|

