|
Infantile hemangiomas (IH)
are the most common infantile tumor, with a
frequency of 4-10% [1]. Recently, there has been
an interest in propranolol and other
beta-blockers in the treatment of IH.
Propranolol may be more effective and safer than
previously established therapies, and may be an
alternative when more widely accepted treatments
for IH have failed. Initial studies suggest that
it may also be used as a first-line therapy.
Other selection criteria may include lesion
location that is inaccessible to surgery,
lesions with a deep component, severe ulceration
and/or cosmetic disfigurement, obstruction of
airway or visual axis, and the presence of
contraindications to other medical therapies.
Parental apprehension remains an important
indication for treatment in cutaneous IH,
irrespective of the possibility of spontaneous
involution. In this review, the authors
summarize the existing literature concerning
propranolol use in IH treatment and provide
suggestions for its clinical use and for future
areas of study.
Natural History
IH are benign tumors of the
vascular endothelium. The first sign of IH is
characteristically an area of pallor that
appears several days after birth. The
proliferative phase of hemangioma development is
characterized by excessive angiogenesis and
occurs over three to six months, whereas the
involutional phase occurs over several years.
Although complete involution occurs over months
to years [1], IH may remain unresolved. Up to
40% of lesions result in textural changes and
fibrofatty scarring [2].
Diagnosis
A vascular lesion that
follows the pattern described is taken to be IH
until proven otherwise [2]. If a lesion does not
follow the established pattern, diagnosis can be
supported by Doppler ultrasound, magnetic
resonance imaging (MRI) or angiography [1].
Locations and Complications
Cutaneous IH can be
cosmetically disfiguring and may cause parental
apprehension. About 5-13% of cutaneous IH
ulcerate [1], which may lead to pain, bleeding
and scarring. Glottic IH can extend to the
supra-glottic and sub-glottic regions and can
partially occlude the airway [3]. Periorbital IH
may lead to vision deprivation, astigmatism and
strabismus [4]. Involvement of the ear canals
may impede hearing. IH involving the viscera or
central nervous system may be complicated by
hemorrhage, thrombocytopenia, anemia,
disseminated intravascular coagulation and
congestive cardiac failure, a constellation of
symptoms collectively described as
Kasabach-Meritt syndrome. Extensive IH may occur
in PHACE syndrome (posterior fossa
malformations, hemangiomas, arterial lesions,
coarctation of the aorta and other cardiac
defects, and eye abnormalities) [1].
Pathophysiology
IH are clonal expansions of
endothelial cells. Genetic mutations in
angiogenesis-related protein expression have
been identified [5]. Characteristic features of
IH include elevated expression of alkaline
phosphatase, factor VIII antigen, and cluster of
differentiation 31. Elevated expression of pro-angiogenic
factors, such as vascular endothelial growth
factor (VEGF), basal fibroblast growth factor
and proliferating cell nuclear antigen may
stimulate endothelial growth and lead to
dysregulated angiogenesis [6]. The
overexpression of glucose transporter 1 (GLUT-1)
is specific to IH and is not found in other
vascular tumors [7].
IH Treatment Patterns and
Modalities
Treatment for IH may be
necessary to a) prevent or improve functional
impairment or pain, b) prevent or improve
scarring or disfigurement, and c) avoid
life-threatening complications. Accepted
treatments include intralesional and systemic
corticosteroids, chemotherapy (vincristine and
interferon alpha), liquid nitrogen cryotherapy,
laser ablation and surgical excision [1].
Corticosteroids are currently the preferred
therapy for most types of IH.
Recent interest in the use of
propranolol in the treatment of IH followed a
2008 report by Ležautež-LabreŐze following an
incidental finding [8]. Following several
corroborative reports, propranolol therapy was
further investigated in a twenty-patient
randomized control trial (RCT) [9].
Mechanism of Action
Several mechanisms of action
for propranolol have been suggested. Results
from combined grayscale and color Doppler
ultrasound imaging suggest that propranolol
reduces vessel density [10]. Propranolol has a
dose-dependent cytotoxic effect on cultured
hemangioma endothelial cells via the
hypoxia-inducible factor 1Š pathway, leading to
decreased secretion of VEGF [11]. In vitro
propranolol decreases plasmalemmal expression of
GLUT-1 [12], though no study has evaluated this
hypothesis to date with respect to IH. Other
possible mechanisms of action include inhibition
of matrix metalloproteinases, down-regulation of
interleukin-6 and modulation of stem-cell
differentiation [11].
Methods
We conducted a search on
PubMed using combinations of search terms "propranolol",
"hemangioma", "beta-blocker," and
"beta-antagonist." All relevant publications
from 2009-2012 in all languages were reviewed.
Bibliographies of relevant articles were
investigated. We reported trial methodology,
dosing and investigations, IH localizations,
follow-up data, and adverse effects from RCTs,
case series, and case reports. Levels of
evidence were determined using Oxford criteria
(www.cebm.org). In table I, level 3 studies and
higher were universally included, and level 4
studies were included if n
≥30.
Studies were assessed for bias.
Results
A number of studies have
evaluated propranolol in IH (Table I).
Most investigators did not specify during which
phase the treatment took place, though one
investigator examined the efficacy of
propranolol after the proliferative phase [14].
A few investigators included patients previously
treated with steroids [4,8,15-19]. Patients with
bronchial asthma were generally excluded. Most
investigators increased drug dose to the target
over days to weeks [3,9,18,20-24], while others
initiated treatment at the target dose [13,15,
25-27]. Some studies used quantitative scores
and/or serial photography to assess IH
regression [9,13,15, 23, 25, 28]. Treatment was
discontinued following either regression or
complete resolution. A few investigators
followed dose-tapering protocols [3,9,25].
Follow-up was done after treatment
discontinuation to monitor for rebound growth.
To summarize the information presented in table
I, dosages of propranolol ranged from 1.0-3.0
mg/kg/day and the duration of treatment ranged
from 1-14 months, the mean duration being 5.85
months. Mean age at treatment was 12.7 months.
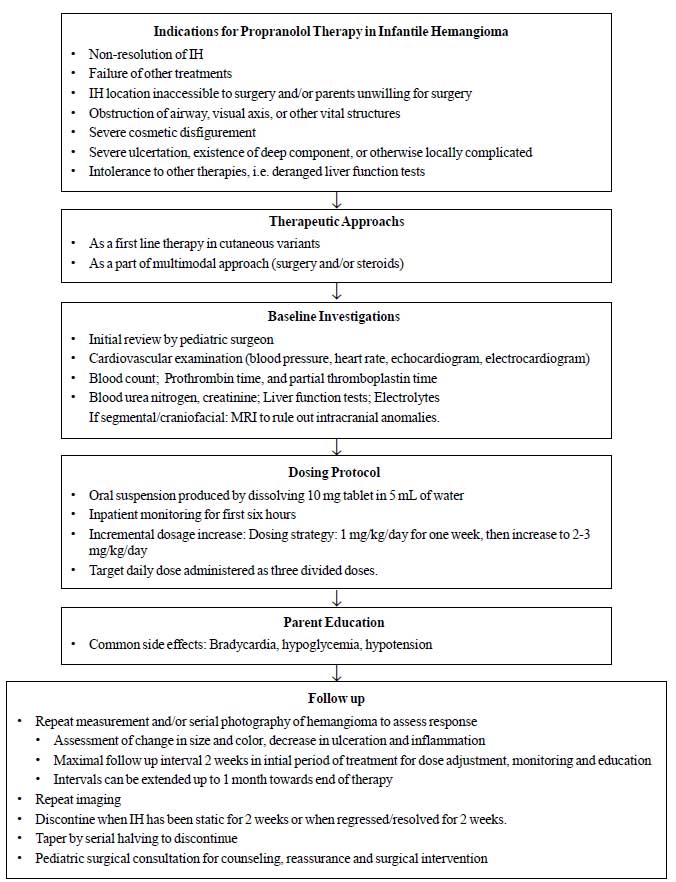 |
|
Fig.1 Suggested
management approach to infantile
hemangioma.
|
Outcomes
A summary of the larger
studies in the literature is provided in
Web Table I.
Cutaneous hemangiomas.
Indications for treatment include extensive
ulceration, presence of deep component, and
cosmetic disfigurement (Fig.1).
Propranolol reduces color intensity, size and
thickness of IH. IH may improve as early as 4
weeks after treatment initiation [15].
Propranolol has been reported to resolve
ulcerated IH [18, 26]. Propranolol has been
documented to resolve IH resistant to steroid
treatment [4,8,15-19].
Hepatic hemangioma.
Propranolol has been documented to resolve
hepatic IH [16]. Therapy can be initiated when
steroids are contraindicated, using an
incremental dose increase and close MRI
monitoring. Periodic ultrasonography can also
document tumor regression [19]. Propranolol in
hepatic IH has been used as late as 10 months
after birth [29].
Orbital hemangioma.
Propranolol treatment is efficacious in the
majority of orbital IH [4], occasionally leading
to resolution of proptosis and reduction of
astigmatism and amblyopia [4,30].
Subglottic hemangioma.
Favorable reports in laryngeal IH have been
published [17, 31]. Periodic laryngoscopy is
required to monitor tumor regression.
Other hemangiomas.
Propranolol has successfully resolved
retroperitoneal [32] and mediastinal [33]
hemangiomas. In reported outcomes in children
with PHACE syndrome, propranolol caused
significant yet incomplete reduction of IH
[3,34-37]. The response to propranolol is
maximal in the first 20 weeks of treatment and
may subside after this [36]. Imaging of cerebral
blood vessels should be undertaken before
treatment to determine the risk of cerebral
ischemia [3].
Limitations. Complete
treatment failure has been documented [3,31]. IH
may undergo rebound growth following cessation
of treatment [8,38].
Adverse Effects
Adverse effects include
hypoglycemia, bradycardia, hypotension, and
airway hyperreactivity. In an RCT, bradycardia
and hypotension were the most common adverse
effects [9]. Hypoglycemia may occur secondary to
inhibited glycogenolysis, glycolysis and
lipolysis [39]. A list of side effects
attributed to propranolol use in infants is
provided in Box I.
Box I Adverse Effects
Associated with Nonselective Beta-Blocker
Therapy
|
Metabolic
Hypoglycemia [22,
39]
Cardiac
Bradycardia [15, 20,
49]
Hypotension [13, 20,
21, 28]
Tachycardia [22]
Central Nervous System
Agitated sleep [9,
15, 18, 21, 26, 28, 30, 49]
Insomnia [13]
Drowsiness [18, 23,
24, 30, 38]
Crying
episodes/Anxiety [30]
Hypotonia [23]
|
Pulmonary
Wheezing [13, 20,
24]
Stridor [15]
RSV exacerbation [9,
50]
Gastrointestinal
Diarrhea/GI upset
[18, 28, 34, 49]
Gastroesophageal reflux [21, 24, 26,
34]
Other
Cold palms [13, 24,
26]
Night sweats [13]
Skin changes [22,
24, 50]
|
Preterm infants
Propranolol was reported to
be effective in a series comprised of seven
preterm infants. No side effects, including
changes in heart rate or blood pressure, were
reported in this group [40]. Similar findings
were reported in a series that included six
preterm infants [41]. Another published report
evaluated propranolol use in 16 very-low birth
weight children, of whom nine were born between
27-34 weeks of gestation. Two infants in these
series had a fall in blood pressure within the
first six hours of therapy, though this was
within physiological limits [42].
Other beta-blockers
Acebutolol, nadolol and
timolol may provide similar efficacy to
propranolol in IH treatment. Acebutolol has been
used in treatment of subglottic IH [31]. Topical
timolol has been effective for cutaneous [20]
and ocular [43] IH. Cardioselective
beta-blockers may be safer and easier to
administer than propranolol.
Combined propranolol and
corticosteroids
In complicated IH,
propranolol may be used in combination with
corticosteroids for a more rapid response. In
one report, a 3-month-old child with a
superficial IH obscuring the visual axis was
treated with combined propranolol and
prednisolone, the latter being gradually
withdrawn over one month [44]. In another
report, two children with airway-obstructing IH
were treated with combined prednisolone and
propranolol. After 24 hours, both children were
relieved of stridor and steroid was discontinued
[45]. These reports suggest that the use of
combination propranolol and corticosteroid
therapy may provide a valid therapeutic approach
to otherwise difficult-to-treat IH.
Propranolol and surgery
Propranolol may limit the
need for surgery in IH. In a multicenter study
comparing propranolol to corticosteroid
treatment for IH, 12% of children treated with
propranolol required surgery, whereas 29% of
those treated with steroids required surgery
[22]. Surgery still serves a role in IH that are
refractory to treatment or that need urgent
treatment [35]. Presumably, in patients with an
incomplete response to propranolol, medical
therapy may limit the extent of surgery
necessary for an easy and cosmetically
acceptable excision.
Route of administration
The route of administration
of propranolol in the majority of published
studies is via oral ingestion. Propranolol is
available in 10mg and 40mg tablets which can be
dissolved in water for easy administration.
Parents should be advised to soak the tablets in
10ml of water for one minute before crushing and
not to filter the suspension [46].
Intralesional drug injection
also causes regression of periorbital IH [47].
The use of a 1% ointment applied for cutaneous
IH led to regression in 85% of patients in a
case series [40]. Topical treatments may result
in fewer adverse effects.
Limitations
Most reviewed studies
provided level 4 evidence. They may be subject
to selection bias. Many of the studies did not
use objective measurement methodologies to
evaluate the efficacy of treatment.
Recommendations
We have suggested a tentative
treatment regimen (Fig.1). The
choice between in-hospital and outpatient
treatment should be made on a case-by-case basis
[48]. The childís age, history of prematurity,
hemangioma subtype and location, comorbidities,
and the level of parental understanding should
be considered. Abdominal ultrasonogram should be
obtained for visceral IH to check for hepatic
artery and portal vein dilatation. Patients with
PHACE syndrome should undergo cerebral
angiography to rule out cerebral ischemia.
Parental education should include discussion of
the warning signs of hypoglycemia and should
emphasize the importance of maintaining a
regular feeding schedule. A pediatric surgical
opinion should be sought, and parents should be
informed of the possibility of surgical excision
if therapy fails. In addition, downgraded IH or
fibrofatty remnants can be excised more
effectively following propranolol therapy.
Special consideration should be made for
premature infants, unusual or high-risk
locations, and patients with Kasabach-Merritt
syndrome. Regression of IH should be monitored
by serial photography. The above protocol should
be evaluated by randomized, large-scale trials.
Conclusion
Case reports have documented
the successful use of propranolol in the Indian
setting [19, 37]. Considering the challenges of
assuring patient compliance and maintaining
close follow-up in India, it is inadvisable to
promote propranolol therapy except in cases
where careful and close monitoring of patient
parameters is feasible.
Propranolol can be tried as
first-line therapy in IH irrespective of age,
location, extent and phase of growth. Treatment
might also be helpful in downgrading the size
and local complications of IH, making the lesion
more amenable to surgical excision. Due to the
lack of long-term side effects and its high
response rate, propranolol therapy may prove to
be superior to existing therapies. More
extensive prospective double-blinded RCT of
propranolol therapy must be carried out and
reported by pediatricians and pediatric surgeons
in order to establish its efficacy conclusively.
Contributors: NG:
designed and conceptualized the study, analyzed
and interpreted the data in the study, and
drafted the manuscript. SR: conceptualized the
study, drafted and revised the manuscript. SB:
conceptualized the study and interpreted the
data. JXS: conceptualized the study, interpreted
the data in the study and revised the
manuscript. All authors gave final approval of
the version to be published.
Funding: None;
Competing interests: None stated.
References
1. Frieden IJ, Haggstrom AN,
Drolet BA, Mancini AJ, Friedlander SF, Boon L,
et al. Infantile hemangiomas: current
knowledge, future directions. Proceedings of a
Research Workshop on Infantile Hemangiomas; 2005
April 7-9; Bethesda, Maryland, USA. Hoboken:
Wiley-Blackwell; 2005.
2. Young AZ, Cohen BA,
Siegfried EC. Cutaneous Congenital Defects.
In: Taeusch HW, Ballard RA, Gleason CA,
Avery ME, editors. Averyís diseases of the
newborn. 8th ed. Philadelphia: Elsevier
Saunders; 2005. p. 1521-38.
3. Manunza F, Syed S, Laguda
B, Linward J, Kennedy H, Gholam K, et al.
Propranolol for complicated infantile
haemangiomas: a case series of 30 infants. Br J
Dermatol. 2010;162:466-8.
4. Cheng JF, Gole GA,
Sullivan TJ. Propranolol in the management of
periorbital infantile haemangioma. Clin
Experiment Ophthalmol. 2010;38:547-53.
5. Yu Y, Varughese J, Brown
LF, Mulliken JB, Bischoff J. Increased Tie2
expression, enhanced response to angiopoietin-1,
and dysregulated angiopoietin-2 expression in
hemangioma-derived endothelial cells. Am J
Pathol. 2001;159:2271-80.
6. Takahashi K, Mulliken JB,
Kozakewich HP, Rogers RA, Folkman J, Ezekowitz
RA. Cellular markers that distinguish the phases
of hemangioma during infancy and childhood. J
Clin Invest. 1994;93:2357-64.
7. North PE, Waner M,
Mizeracki A, Mihm MC, Jr. GLUT1: a newly
discovered immunohistochemical marker for
juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
8. Leaute-Labreze C, Dumas de
la Roque E, Hubiche T, Boralevi F, Thambo JB,
Taieb A. Propranolol for severe hemangiomas of
infancy. N Engl J Med. 2008;358: 2649-51.
9. Hogeling M, Adams S,
Wargon O. A randomized controlled trial of
propranolol for infantile hemangiomas.
Pediatrics. 2011;128:e259-66.
10. Bingham MM, Saltzman B,
Vo NJ, Perkins JA. Propranolol reduces infantile
hemangioma volume and vessel density.
Otolaryngol Head Neck Surg. 2012;147:338-44.
11. Greenberger S, Bischoff
J. Infantile Hemangioma-Mechanism(s) of Drug
Action on a Vascular Tumor. Cold Spring Harb
Perspect Med. 2011;1:a006460.
12. Egert S, Nguyen N,
Schwaiger M. Contribution of alpha-adrenergic
and beta-adrenergic stimulation to
ischemia-induced glucose transporter (GLUT) 4
and GLUT1 translocation in the isolated perfused
rat heart. Circ Res. 1999;84:1407-15.
13. Sans V, de la Roque ED,
Berge J, Grenier N, Boralevi F,
Mazereeuw-Hautier J, et al. Propranolol
for severe infantile hemangiomas: follow-up
report. Pediatrics. 2009;124:e423-31.
14. Zvulunov A, McCuaig C,
Frieden IJ, Mancini AJ, Puttgen KB, Dohil M,
et al. Oral propranolol therapy for
infantile hemangiomas beyond the proliferation
phase: a multicenter retrospective study.
Pediatr Dermatol. 2011;28:94-8.
15. Bagazgoitia L, Torrelo A,
Gutierrez JC, Hernandez-Martin A, Luna P,
Gutierrez M, et al. Propranolol for
infantile hemangiomas. Pediatr Dermatol.
2011;28:108-14.
16. Marsciani A, Pericoli R,
Alaggio R, Brisigotti M, Vergine G. Massive
response of severe infantile hepatic hemangioma
to propanolol. Pediatr Blood Cancer.
2010;54:176.
17. Mistry N, Tzifa K. Use of
propranolol to treat multicentric airway
haemangioma. J Laryngol Otol. 2010;124:1329-32.
18. Hermans DJ, van Beynum
IM, Schultze Kool LJ, van de Kerkhof PC, Wijnen
MH, van der Vleuten CJ. Propranolol, a very
promising treatment for ulceration in infantile
hemangiomas: a study of 20 cases with matched
historical controls. J Am Acad Dermatol.
2011;64:833-8.
19. Muthamilselvan S, Vinoth
PN, Vilvanathan V, Ninan B, Amboiram P, Sai V,
et al. Hepatic haemangioma of infancy:
role of propranolol. Ann Trop Paediatr.
2010;30:335-8.
20. Blatt J, Morrell DS, Buck
S, Zdanski C, Gold S, Stavas J, et al.
beta-blockers for infantile hemangiomas: a
single-institution experience. Clin Pediatr (Phila).
2011;50:757-63.
21. Holmes WJ, Mishra A,
Gorst C, Liew SH. Propranolol as first-line
treatment for rapidly proliferating infantile
haemangiomas. J Plast Reconstr Aesthet Surg.
2011;64:445-51.
22. Price CJ, Lattouf C, Baum
B, McLeod M, Schachner LA, Duarte AM, et al.
Propranolol vs corticosteroids for infantile
hemangiomas: a multicenter retrospective
analysis. Arch Dermatol. 2011;147:1371-6.
23. Rossler J, Schill T, Bahr
A, Truckenmuller W, Noellke P, Niemeyer CM.
Propranolol for proliferating infantile
haemangioma is superior to corticosteroid
therapy - a retrospective, single centre study.
J Eur Acad Dermatol Venereol. 2012;26:1173-5.
24. Schupp CJ, Kleber JB,
Gunther P, Holland-Cunz S. Propranolol therapy
in 55 infants with infantile hemangioma: dosage,
duration, adverse effects, and outcome. Pediatr
Dermatol. 2011;28:640-4.
25. Celik A, Tiryaki S,
Musayev A, Kismali E, Levent E, Ergun O.
Propranolol as the first-line therapy for
infantile hemangiomas: preliminary results of
two centers. J Drugs Dermatol. 2012;11:808-11.
26. Saint-Jean M,
Leaute-Labreze C, Mazereeuw-Hautier J, Bodak N,
Hamel-Teillac D, Kupfer-Bessaguet I, et al.
Propranolol for treatment of ulcerated infantile
hemangiomas. J Am Acad Dermatol. 2011;64:827-32.
27. Talaat AA, Elbasiouny MS,
Elgendy DS, Elwakil TF. Propranolol treatment of
infantile hemangioma: clinical and radiologic
evaluations. J Pediatr Surg. 2012;47:707-14.
28. Bertrand J, McCuaig C,
Dubois J, Hatami A, Ondrejchak S, Powell J.
Propranolol versus prednisone in the treatment
of infantile hemangiomas: a retrospective
comparative study. Pediatr Dermatol.
2011;28:649-54.
29. Mazereeuw-Hautier J,
Hoeger PH, Benlahrech S, Ammour A, Broue P, Vial
J, et al. Efficacy of propranolol in
hepatic infantile hemangiomas with diffuse
neonatal hemangiomatosis. J Pediatr.
2010;157:340-2.
30. Schiestl C, Neuhaus K,
Zoller S, Subotic U, Forster-Kuebler I, Michels
R, et al. Efficacy and safety of
propranolol as first-line treatment for
infantile hemangiomas. Eur J Pediatr.
2011;170:493-501.
31. Blanchet C, Nicollas R,
Bigorre M, Amedro P, Mondain M. Management of
infantile subglottic hemangioma: acebutolol or
propranolol? Int J Pediatr Otorhinolaryngol.
2010;74:959-61.
32. Vanlander A, Decaluwe W,
Vandelanotte M, Van Geet C, Cornette L.
Propranolol as a novel treatment for congenital
visceral haemangioma. Neonatology.
2010;98:229-31.
33. Fulkerson DH, Agim NG,
Al-Shamy G, Metry DW, Izaddoost SA, Jea A.
Emergent medical and surgical management of
mediastinal infantile hemangioma with
symptomatic spinal cord compression: case report
and literature review. Childs Nerv Syst.
2010;26:1799-805.
34. Haider KM, Plager DA,
Neely DE, Eikenberry J, Haggstrom A. Outpatient
treatment of periocular infantile hemangiomas
with oral propranolol. J AAPOS. 2010;14:251-6.
35. Leboulanger N, Cox A,
Garabedian EN, Denoyelle F. Infantile
haemangioma and beta-blockers in otolaryngology.
Eur Ann Otorhinolaryngol Head Neck Dis.
2011;128:236-40.
36. Hernandez-Martin S,
Lopez-Gutierrez JC, Lopez-Fernandez S, Ramirez
M, Miguel M, Coya J, et al. Brain
Perfusion SPECT in Patients with PHACES Syndrome
Under Propranolol Treatment. Eur J Pediatr Surg.
2011.
37. Mohanan S, Besra L,
Chandrashekar L, Thappa DM. Excellent response
of infantile hemangioma associated with PHACES
syndrome to propranolol. Indian J Dermatol
Venereol Leprol. 2012;78:114-5.
38. Chai Q, Chen WL, Huang
ZQ, Zhang DM, Fan S, Wang L. Preliminary
Experiences in Treating Infantile Hemangioma
With Propranolol. Ann Plast Surg. 2011.
39. Holland KE, Frieden IJ,
Frommelt PC, Mancini AJ, Wyatt D, Drolet BA.
Hypoglycemia in children taking propranolol for
the treatment of infantile hemangioma. Arch
Dermatol. 2010;146:775-8.
40. Kunzi-Rapp K. Topical
propranolol therapy for infantile hemangiomas.
Pediatr Dermatol. 2012;29:154-9.
41. Moehrle M, Leaute-Labreze
C, Schmidt V, Rocken M, Poets CF, Goelz R.
Topical Timolol for Small Hemangiomas of
Infancy. Pediatr Dermatol. 2012.
42. Erbay A, Sarialioglu F,
Malbora B, Yildirim SV, Varan B, Tarcan A, et
al. Propranolol for infantile hemangiomas: a
preliminary report on efficacy and safety in
very low birth weight infants. Turk J Pediatr.
2010;52:450-6.
43. Ye XX, Jin YB, Lin XX, Ma
G, Chen XD, Qiu YJ, et al. [Topical timolol in
the treatment of periocular superficial
infantile hemangiomas: a prospective study].
Zhonghua Zheng Xing Wai Ke Za Zhi.
2012;28:161-4.
44. Koay AC, Choo MM, Nathan
AM, Omar A, Lim CT. Combined low-dose oral
propranolol and oral prednisolone as first-line
treatment in periocular infantile hemangiomas. J
Ocul Pharmacol Ther. 2011;27:309-11.
45. Rosbe KW, Suh KY, Meyer
AK, Maguiness SM, Frieden IJ. Propranolol in the
management of airway infantile hemangiomas. Arch
Otolaryngol Head Neck Surg. 2010;136:658-65.
46. Nunn A, Shah U, Ford J.
Giving propranolol tablets to infants with
hemangiomas. J Paediatr Child Health.
2011;47:927.
47. Awadein A, Fakhry MA.
Evaluation of intralesional propranolol for
periocular capillary hemangioma. Clin Ophthalmol.
2011;5:1135-40.
48. Lawley LP, Siegfried E,
Todd JL. Propranolol treatment for hemangioma of
infancy: risks and recommendations. Pediatr
Dermatol. 2009;26:610-4.
49. Qin ZP, Liu XJ, Li KL,
Zhou Q, Yang XJ, Zheng JW. [Treatment of
infantile hemangiomas with low-dose propranolol:
evaluation of short-term efficacy and safety].
Zhonghua Yi Xue Za Zhi. 2009;89:3130-4.
50. Buckmiller LM, Munson PD,
Dyamenahalli U, Dai Y, Richter GT. Propranolol
for infantile hemangiomas: early experience at a
tertiary vascular anomalies center.
Laryngoscope. 2010;120:676-81.
|

