|
|
|
Indian Pediatr 2010;47: 241-244 |
 |
Effect of Albumin Administration Prior to
Exchange Transfusion in Term Neonates with Hyperbilirubinemia –
A Randomized Controlled Trial |
|
Mozhgan Shahian and Mohammad Ashkan
Moslehi
From the Division of Neonatology, Department of
Pediatrics, Shiraz University of Medical Sciences, Shiraz, Iran.
Correspondence to: Dr Mozhgan Shahian, Assistant
Professor, Division of Neonatology, Department of Pediatrics, Shiraz
University of Medical Sciences, Shiraz, Iran. [email protected]
Received: June 24, 2008;
Initial review: July 23, 2008;
Accepted: March 18, 2009.
Published online 2009 May 20.
PII:S097475590800395-1
|
|
Abstract
Objective: To determine the role of intravenous
administration of human albumin prior to blood exchange in term neonates
for reduction of total serum bilirubin (TSB).
Design: Randomized controlled trial.
Setting: Neonatal Unit of Nemazee Hospital,
affiliated with Shiraz University of Medical Sciences, southern Iran.
Patients: Fifty out-born term neonates with
gestation age >37 weeks, birth weight >2500 g, otherwise healthy with
TSB ³25 mg/dL requiring blood
exchange due to intensive phototherapy failure.
Intervention: Intervention group (n=25)
received intravenous human albumin 20% (1 g/kg) one hour before exchange
while the control group (n=25) underwent a blood exchange.
Outcome Measures: TSB level at 6 and 12 hours
post-exchange, total duration of phototherapy, need for a second
exchange transfusion and adverse effects.
Results: The mean TSB level in albumin-treated
group was significantly lower than that in the control group at 6 and 12
hours post-exchange (P<0.001). Mean duration of phototherapy was
significantly reduced in the albumin-treated group, compared to that in
the control group (8.6±2.4 vs. 25±8.2 hours) (P<0.001).
None of the neonates in albumin-treated group needed exchange
transfusion again and no side effects were observed.
Conclusion: Infusion of 20% albumin (1 g/kg) one
hour prior to blood exchange can significantly reduce the post-exchange
total serum bilirubin and duration of phototherapy.
Key words: Albumin, Exchange transfusion, Hyper-bilirubinemia,
Management, Neonate.
|
|
R
apid reduction in serum unbound
bilirubin may be theoretically effective for the prevention of bilirubin
encephalopathy. Bilirubin is bound to albumin as the dianion with a
primary binding site that has a capacity of binding of one molecule of
bilirubin. A molar ratio of 1.0 indicates that approximately 8.3 mg
bilirubin is bound to each 1 g albumin(1). From a therapeutic viewpoint,
albumin infusion may be advantageous, because an increased reserve of
albumin may be protective against bilirubin toxicity by providing more
binding sites, thereby reducing the levels of unbound bilirubin(2).
Intensive phototherapy for severe hyper-bilirubinemia may cause
photo-oxidation of albumin, resulting in a decrease or disappearance of
its binding affinity for bilirubin(3). Accordingly, albumin infusion
therapy might be effective on unbound-bilirubin values in term neonates
with intensive photo-therapy(4). Exchange transfusion is indicated for
severe jaundice when other therapeutic modalities have failed(5). The
present study aims at investigating the effect of intravenous
administration of human albumin prior to exchange transfusion on reduction
of total serum bilirubin levels (TSB).
Methods
This randomized controlled study was conducted between
February and July 2006 on 50 outborn neonates with jaundice admitted to
the Neonatal Unit of Nemazee Hospital, affiliated with Shiraz University
of Medical Sciences, southern Iran. Term neonates (gestational age more
than 37 weeks) with birthweight >2500 g with TSB
³25
mg/dL, requiring blood exchange due to intensive phototherapy failure and
otherwise healthy, entered our study. "Healthy" was defined as an active
neonate on oral feed with normal neurological findings and physiological
vital parameters. We excluded neonates with hemolytic diseases (Rh or ABO
incompatibility and a positive Coombs’ test), infectious diseases
(congenital or acquired), G6PD deficiency and direct hyperbilirubi-nemia
(conjugated bilirubin >1.5 mg/dL and 10% of TSB). These parameters were
checked prior to randomization.
Assuming the least expected difference to be 6 mg/dL
between intervention and control groups and the standard deviation of 1.5,
a two sided alpha of 0.05 and power of 0.9 ( b
= 0.1) with equal allocation,
the estimated sample size would be 42 (21 neonates in each group). To
avoid loss to follow up, we enroled 25 neonates in each group. Enroled
neonates were randomized into intervention and control groups based on
simple randomization. The random numbers were computer generated and slips
bearing the allocated group were placed in serially numbered, opaque,
sealed envelopes. The primary outcome was the TSB level at 6 and 12 hours
post exchange. Secondary outcomes were the total duration of phototherapy,
need for a second exchange transfusion and adverse effects (respiratory
distress, edema, etc).
All neonates received intensive phototherapy using 8
special blue tube lamps (Philips TL 20 W/52) positioned within 15 to 20 cm
of the patient’s body. Irradiance was checked by a photoradiometer to
maintained approximately 20
mw/nm/cm2
at all times. Blood exchange transfusions were done for the
above-mentioned neonates due to intensive photo-therapy failure defined
as, the inability to produce a decline of 1 to 2 mg/dL within 4 hours
after the initiation of phototherapy(6). Prior to the exchange, complete
blood count, blood group typing of neonates and mothers, direct Coombs
test, reticulocyte count, albumin and serum bilirubin levels (total and
direct) were performed and all information regarding demo-graphic data
were recorded. Twenty five neonates in intervention group received
intravenous 20% human albumin (Biotest, Germany) within one hour, with a
dose of 1g/kg, one hour before exchange, while the control group only
underwent a blood exchange. TSB was measured every 6 hours for both groups
during the first 24 hours following the exchange using a Unistat®
bilirubinometer (Reichert-Germany). All the infants were examined 2 days
after discharge in outpatient clinic for further evaluation of their
jaundice and any side effects of the drug.
Statistical analysis: The data obtained were
analyzed using SPSS software version 11.5 for Windows. Numerical variables
were compared between the two groups by using the independent student’s
test. The Chi-square test was used to compare sex and route of delivery
between the two groups. P values of less than 0.05 were considered
as statistically significant.
Informed consent was obtained from the parents and the
study protocol was approved by the University Ethical Committee.
Results
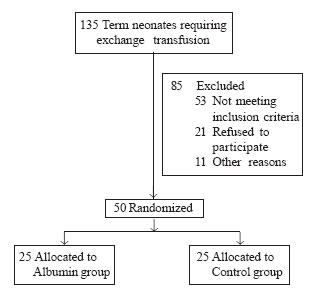
Of 135 term neonates with TSB >25 mg/dL that received
intensive phototherapy and required exchange transfusion, 50 infants who
satisfied the eligibility criteria were enrolled in the study and were
randomized (Fig.1). Baseline demographic charac-teristics
were comparable between the two groups (Table I).
TABLE I
Demographic Characteristics and Laboratory Data of Patients at Admission
|
Parameters |
Albumin group |
Control group |
P |
| |
mean ±
SD |
mean ±
SD |
value |
| Gestation (wk) |
39.3±1.2 |
39.5±1.5 |
0.6 |
| Birthweight (g) |
3239±585 |
3264±428 |
0.86 |
| Cesarean section |
7 |
8 |
0.75 |
| Apgar at 1 min |
8.6±1.2 |
8.8±1.3 |
0.57 |
| Age (d) |
7±1.1 |
8±1.0 |
0.001 |
| Albumin (g/dL) |
3.4±0.4 |
3.5±0.6 |
0.49 |
| pH |
7.40±0.04 |
7.41±0.05 |
0.43 |
| TSB (mg/dL) |
30±3.64 |
29±3.65 |
0.34 |
| Direct bilirubin (mg/dL) |
0.5±0.30 |
0.4±0.35 |
0.28 |
| Sex (female) (%) |
12 (48) |
13 (52) |
0.777 |
 |
|
Fig. 1. Study flow chart. |
Following double blood volume exchange, TSB was
measured every 6 hours, The mean TSB in albumin-treated group was
statistically lower than that in the control group at 6 and 12 hours post
exchange. Baseline albumin level and its level at 24 hours after exchange
were compared and there was no significant difference between the mean of
serum albumin levels in the two groups. No neonate in albumin-treated
group required phototherapy after 12 hours, but 8 (32%), 13 (52%), and 4
neonates (16%) in the control group received phototherapy till 18, 24 and
36 hours post-exchange, respectively. The difference between the duration
of phototherapy in albumin-treated group and the control group was
statistically significant (P<0.001) (Table II).
TABLE II
Outcome in the Two Groups
|
Variables |
Albumin-treated |
Control group |
P |
| |
group(n=25) |
(n=25) |
value |
| TSB
levels after 6 h (mg/dL) |
14.4 ± 1.7 |
21.7±3.2 |
<0.001 |
| TSB
levels after 12 h (mg/dL) |
8 ± 1.5 |
16.1±2.1 |
<0.001 |
| Albumin
level at 24 h (g/dL) |
3.5±0.5 |
3.4±0.3 |
0.39
|
| Duration
of
phototherapy(h) |
8.6±2.4 |
25±8.2 |
<0.001 |
|
*TSB: total serum bilirubin. |
On serial examination during hospitalization and two
days after discharge in the outpatient clinic, no rise was observed in the
TSB levels and no side effects were evident. None of the neonates in
albumin-treated group needed exchange transfusion again but four neonates
in the control group underwent a second exchange due to the relapse of
severe hyperbilirubinemia.
Discussion
Our results suggest that administration of albumin 20%
(1 g/kg) to neonates one hour prior to the exchange transfusion increases
the efficiency of bilirubin removal by shifting more tissue-bound
bilirubin into the circulation and significantly reduces the post-exchange
TSB level and the duration of phototherapy.
There have been insufficient studies to determine the
effect of albumin infusion on TSB level along with a double volume blood
exchange but there are some studies about albumin administration combined
with phototherapy in the treatment of hyperbilirubinemia. Hosono, et al.(7)
showed that albumin priming may be effective for an immediate reduction in
serum unbound bilirubin values.
Albumin infusion prior to exchange transfusion
decreases the unbound bilirubin in the intravascular space and due to
equilibration between plasma bili-rubin and extravascular space, more
bilirubin would shift from tissue to plasma. Tsao and Yu(8) have reported
that there was a marked increase in total intravascular bilirubin as well
as plasma volume after priming with albumin. Therefore, more bilirubin
would be removed through exchange transfusion leading to a decrease in
total body bilirubin concentration. So, the rebound of plasma bilirubin in
post-exchange would increase less in albumin-treated group. The present
study was not able to determine the unbound bilirubin level and its
changes during the albumin infusion, because the measurement of free
bilirubin level was not possible in the studied center. We selected
otherwise healthy term neonates to reduce the risk of alterations in blood
brain barrier permeability because theoretically, the transient increase
in TSB concentration after albumin administration may increase the risk of
kernicterus if the barrier is disrupted with some predisposing factors in
sick neonates(6).
We also demonstrated that there was a significant
difference in the reduction of TSB levels in albumin-treated group
compared to the control group at 6 and 12 hours post-exchange (P<0.001).
Also, the duration of phototherapy and the risk of second exchange
transfusion were reduced in the former. There was no significant
difference between the baseline albumin level and its level at 24 hours
post-exchange in albumin-treated group and the same result was reported by
Hosono, et al.(7). The unchanged albumin level may be due to the
generated plasma oncotic pressure induced by albumin infusion that draws
fluid out of the extravascular into vascular space and the dilution effect
of the expanded plasma volume.
Acknowledgments
We thank the Office of Vice Chancellor for Research of
Shiraz University of Medical Sciences for financial support to this study
and Dr Davood Mehrabani, Mrs Ghorbani and Miss Gholami at the Center for
Development of Clinical Research of Nemazee. Our special thanks to H
Khajehei at PACMRC for his invaluable linguistic copy editing.
Contributors: Both authors contributed to
the study design, analytical framework for the study, performing the final
data analysis and writing the manuscript.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Treatment with albumin prior to exchange
transfusion is not routinely recommended.
What This Study Adds?
• Albumin infusion prior to exchange transfusion
in term neonates can effectively decrease the total serum bilirubin
without any side effects.
|
References
1. Ebbesen F, Jacobsen J. Bilirubin- albumin binding
affinity and serum albumin concentration during intensive phototherapy
(blue double light) in jaundiced newborn infants. Eur J Pediatr 1980; 134:
261-263.
2. Porter EG, Waters WJ. A rapid micromethod for
measuring the reserve albumin binding capacity in serum from newborn
infants with hyper-bilirubinemia. J Lab Clin Med 1966; 67: 660-668.
3. Wood B, Comley A, Sherwell J. Effect of additional
albumin administration during exchange trans-fusion on plasma
albumin-binding capacity. Arch Dis Child 1970; 45: 59-62.
4. Hosono S, Ohno T, Kimoto H, Nagoshi R, Shimizu M,
Nozawa M. Follow-up study f auditory brainstem responses in infants with
high unbound bilirubin levels treated with albumin infusion therapy.
Pediatr Int 2002; 44: 488-492.
5. Jahnson LH, Brown AK, Bhutani VK. System-based
approach to management of neonatal jaundice and prevention of Kernicterus.
J Pediatr 2002; 140: 397-386.
6. Halamek LP, Stevenon DK. Neonatal jaundice and liver
disease. In: Fanaroff AA, Martin RJ, editors. Neonatal-Perinatal Medicine.
7th ed Philadelphia: Mosby Publishers & Distributors; 2002. p. 1334-1335.
7. Hosono S, Ohno T, Kimoto H, Nagoshi R, Shimizu M,
Nozawa MH. Effects of albumin infusion therapy on total and unbound
bilirubin values in term infants with intensive phototherapy. Pediatr Int
2001; 43: 8-10.
8. Tsao YC, Yu VYH. Albumin in management of neonatal
hyperbilirubinemia. Arch Dis Child 1972; 47: 250- 252.
|
|
|
 |
|

