Ewing sarcoma is the second most common primary bone cancer
affecting mainly adolescents in the second decade of their life
[1]. It has a predilection for long bones (47%), pelvis
(26%), chest wall (16%) and spine (6%) [2]. Pain is the most
common initial symptom as with other bone sarcomas [3]. Ewing
sarcoma is highly metastatic; although, it can be locally
controlled by radiotherapy or surgery, historically, 85%-90% of
patients die within a few months from a metastasis without
systematic treatment neither before nor after local treatment
[4]. After the addition of doxorubicin to vincristine,
actinomycin D, and cyclophosphamide (VACD regimen), the 5-year
overall survival rate of local disease increased from 28% to 65%
in the 1970s [5]. Chemotherapy was initially used as systematic
treatment to control metastasis, and later in a neoadjuvant
setting to enhance local control with confirmed efficacy [6].
Local control is an
important method to improve the overall survival rate and local
control rate of Ewing sarcoma patients. Local treatment is
recommended after chemotherapy for all patients. Current local
control strategies include isolated radiotherapy, isolated
surgery, or combined surgery and radiotherapy [7]. The debate
over whether surgery and radiotherapy are comparable in terms of
local control continues [8]. The optimal local control strategy
for Ewing sarcoma remains unclear. The French association for
pediatric research suggested that surgery or surgery combined
with radiotherapy is the best local treatment for pelvic tumors,
while radiotherapy is only available to patients who cannot
undergo surgery or patients who are resistant to chemotherapy,
or surgery involves amputation [10,11]. Zogopoulos, et al.
[12] suggested that surgery is the most effective method for
local treatment, while radiotherapy should be used sparingly.
Moreover, with the neoadjuvant application of chemotherapy, we
are still looking for a conclusive analysis concerning whether
surgery and radiotherapy are comparable in terms of local
control. This network meta-analysis aimed at comparing the
efficacy of local control strategies, including surgery,
radiotherapy and combined treatment with radiotherapy and
surgery after neoadjuvant chemotherapy in Ewing sarcoma
patients.
METHODS
PubMed and Embase
database were searched from inception through July 30, 2018,
using controlled vocabulary supplemented with keywords
describing Ewing sarcoma and neoadjuvant chemotherapy. Possible
related studies were also manually identified by screening a
reference list of retrieved articles. Two reviewers
independently primarily evaluated the eligibility of retrieved
articles by screening their titles and abstracts. Disagreement
was resolved by discussion. Subsequently, full text of eligible
articles was reviewed according to inclusion criteria. The
included documents fulfilling the following criteria were
eligible for our analysis: (i) patients were diagnosed
with Ewing sarcoma, and tumors were clinically diagnosed as
operable and non-metastatic; (ii) all the patients were
treated with neoadjuvant chemotherapy; (iii) efficacy of
at least two of three investigated local control strategies,
i.e. surgery, radiotherapy, and surgery combined with
radiotherapy, should be compared in the clinical trial, and all
treatments for local control were performed after neoadjuvant
chemotherapy; and (iv) available data was sufficient for
further analysis. Furthermore, trials were excluded for
duplicates, articles based on the same clinical trials, and
those not reported in English. We applied Cochrane
collaboration’s tool for assessing risk of bias [13] to evaluate
the quality of enrolled randomized clinical trials, and
Methodological index for non-randomized studies (MINORS) for the
quality of randomized trials [14].
In our analysis, we used 5-year
local recurrence rate (5-LR) and 5-year event-free survival rate
(5-EFSR) as outcomes of investigated treatment. Considering that
the main evaluation method of Ewing sarcoma is local recurrence
rate, and the survival data is relatively lacking, we used the
local recurrence rate as the main outcome index and the survival
data as the secondary outcome index.
Relevant data were extracted by two
authors independently and discrepancies were dealt by
discussion. General information including first author, year of
publication, nationality of subjects, study design, sample size
and treatment were documented. Odd ratios (ORs) for OS and EFS
were either extracted from original articles as the summary
statistics or estimated indirectly from survival curve or using
other available information.
Statistical analyses: This
meta-analysis was performed according to the guidelines of
PRISMA with Bayesian model in WinBUGS (MRC Bio-statistics Unit,
Cambridge, UK) for network meta-analysis and STATA 12.0 (Stata
Corp, College Station, TX) for other analyses. For survival
analysis, ORs and the associated 95% credible intervals (CrI)
were used to describe the efficacy of different intervention on
5-LR and 5-EFSR. Surface under the cumulative ranking curve
(SUCRA) was calculated in order to compare the relative ranking
of different therapies. Publication bias was assessed using Begg
and Egger tests. A P value less than 0.05 indicated the
presence of publication bias. A two-side P value less
than 0.05 was considered as significant.
RESULTS
As illustrated in the flow diagram (Fig. 1), a
total of 1170 articles were retrieved from the databases, and
three more records were obtained from other sources. Finally, 11
studies [7-10, 15-21] from 1999 to 2017 were included in our
analysis (Table I). The quality of included
studies was evaluated and they were all well-designed and
reported reliable results. A total of 2540 patients were
enrolled in the meta-analysis in total.
 |
| Fig. 1 Flow diagram
summarizing results of study identification and
selection. |
Table I Details of Studies Included in the Meta-analysis
|
Author, year |
Country |
Design |
Follow-up (y) |
Male |
Age |
N |
Neo-CT |
Intervention |
|
Reporting only LR
recurrence rate |
|
Ahmed [9], 2017 |
US |
Retrospective |
8.3 |
69.0% |
20 (6.6-64.9) |
23 |
VDC/IE 41/Other 7 |
Surgery vs. RT vs. SR |
|
Ahmed [15], 2017 |
US |
Cohort |
NR |
55.0% |
13 (0.5-45) |
956 |
IE based |
Surgery vs. RT vs. SR |
|
Laitinen [16], 2016 |
UK |
Retrospective |
5.2 |
51.1% |
12.4(2-16) |
88 |
NR |
Surgery vs. RT vs. SR |
|
Shankar [21], 1999 |
UK |
Retrospective |
5.5 |
55.8% |
12 (1-27) |
190 |
IVAD |
Surgery vs. RT vs. SR |
|
Carrie [10], 1999 |
France |
Retrospective |
6.5 |
50.7% |
12.9 |
53 |
EW-85/88/9 |
Surgery vs. RT vs. SR |
|
Reporting only EFSR |
|
Grevener [17], 2016 |
Germany |
Retrospective |
10 |
54.0 |
11.5 (3-66) |
43 |
VIDE |
Surgery vs. RT vs. SR |
|
Reporting both LR and EFSR |
|
Donati [8], 2007 |
Italy |
Retrospective |
7.3 |
47.1 % |
18.4 (6-46) |
66 |
CNR/ISG |
RT vs. SR |
|
Dubois [7], 2014 |
US |
Cohort |
NR |
54.4% |
12.4 (0.7-33) |
465 |
VDC/IE |
RT vs. SR |
|
Bacci [18], 2009 |
Italy |
Retrospective |
15 |
62.0% |
17.9 (3-40) |
55 |
IOR |
Surgery vs. RT vs. SR |
|
Yock [19], 2006 |
US |
Retrospective |
4.4 |
52.0% |
NR |
75 |
VACA/VACA-IE |
Surgery vs. RT vs. SR |
|
Bacci [20], 2006 |
Italy |
Retrospective |
12 |
64.1% |
NR |
512 |
REN |
RT vs. SR |
|
CT: Chemotherapy; RT: radiotherapy; SR: Surgery combined with Radiotherapy; VDC/IE, Vincristine, Doxorubicin, Cyclophosphamide, and Ifosfamide, Etoposide; LR: Local recurrence rate; EFSR: Event-free surival rate. |
In the included studies, all patients received neoadjuvant
chemotherapy prior to the investigated local control strategies.
Three different strategies, surgery, radiotherapy and surgery
combined with radiotherapy (SR), were evaluated in the included
studies (Table I).
Web Fig. 1 shows the net plot
of the qualified comparison enrolled in our analysis. The width
of the line represents the cumulative number of trials per
comparison; the circled area represents the cumulative number of
patients per intervention. For the outcomes 5-LR and 5-EFSR, the
comparison between radiotherapy and SR was the most commonly
reported one.
Table II Network Meta-analysis Results for 5-LR and 5-EFSR in Ewing Sarcoma
|
Trials |
OR (95% CrI) |
|
5 LR: No. of arms=28, Patients=2474 | | |
|
*SG vs. RD |
9 |
0.49 (0.30-0.82) |
|
SG vs. SR |
9 |
0.94 (0.56-1.72) |
|
*RD vs. SG |
9 |
2.05 (1.22-3.32) |
|
*RD vs. SR |
10 |
1.95 (1.17-3.32) |
|
SR vs. SG |
9 |
1.06 (0.58-1.79) |
|
*SR vs. RD |
10 |
0.51 (0.30-0.85) |
|
SG vs. RD |
6 |
1.25 (0.41-3.82) |
|
5-EFSR: No. of arms=19, Patients=749 | | |
|
SG vs. SR |
6 |
1.28 (0.39-3.86) |
|
RD vs. SG |
6 |
0.8 (0.26-2.46) |
|
RD vs. SR |
7 |
1.03 (0.35-2.86) |
|
SR vs. SG |
6 |
0.78 (0.26-2.53) |
|
SR vs. RD |
7 |
0.97 (0.35-2.89) |
|
5-LR: 5 year local recurrence rate; 5-EFSR: 5 year event-free survival rate; OR (95% CrI) odds ratio (95% Credible interval); SG: surgery; RD, radiation therapy; SR, surgery combined with radiation; *P<0.03. |
Local recurrence rate: The efficacy of
different interventions was obtained by the use of a network
meta-analysis. A total of 2474 patients from 9 clinical trials
were involved in our analysis. As illustrated in Fig.
2 and Table II, surgery and SR showed no
statistical difference in 5-LR. However, both surgery and SR had
statistically significant differences with radiotherapy.
Compared with radiotherapy, surgery had better efficacy [OR (95%
CI) 0.48 (0.33 -0.87)] and SR had a similar effect with surgery
[OR (95% CI) 0.50 (0.29 -0.82)]. Surgery and SR could
significantly reduce 5-LR of patients who received neoadjuvant
chemotherapy.
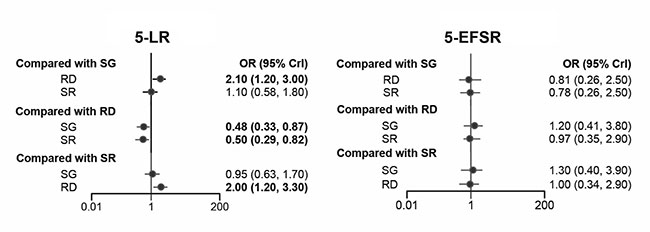 |
| Fig. 2 Five-year
local recurrence rate and 5-year event-free survival
rate in Ewing Sarcoma. |
The SUCRA values show the relative efficacy of different
strategies (Fig. 3). Surgery and SR ranked the
highest for 5-LR (SUCRA value 0.79 and 0.70, respectively).
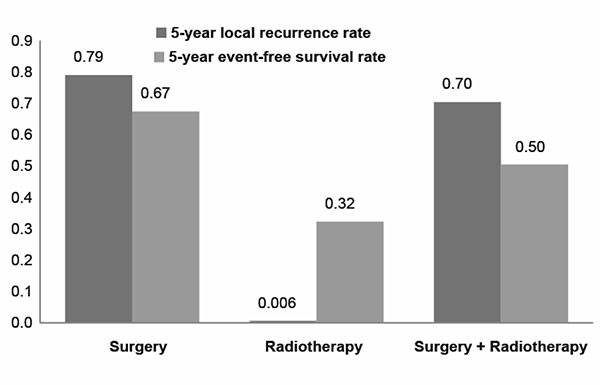 |
| Fig. 3 Surface under the cumulative
ranking curve (SUCRA) of all treatments. Each column
shows the probability of that treatment being ranked at
the top. |
Survival analysis: 749 patients from
seven clinical trials were included in the analysis. There were
no statistically significant differences between the three local
control strategies in 5-EFSR (Fig. 2 and
Table 2). As per SUCRA values, surgery ranked the
highest for 5-EFSR.
Radiotherapy and SR had lower SUCRA values for improving 5-EFSR
(0.32 and 0.50, respectively).
The publication
bias of various studies for 5-LR and 5-EFSR is shown in
Web Fig. 2.
DISCUSSION
In the present meta-analysis, radiotherapy was the least
favorable for improving the prognosis of Ewing sarcoma patients,
with significantly higher 5-LR when compared with SR and
surgery. No significant difference was observed between surgery
and SR, yet the SUCRA value indicated that surgery had higher
ranking probability on decreasing 5-LR.
Surgery, radiotherapy and SR showed no significant
difference of 5-EFSR, while surgery ranked the highest as per
ranking probabilities by SUCRA value.
DuBois, et al. [7] reported that radiation had a higher
risk of local failure, when compared with that of localized ES
patients treated with surgery. A study conducted by Bacci, et
al. [20] showed that the recurrence rate after radiation
therapy was high in patients with ES family tumors. In addition,
the risk of second malignancies was another significant
consideration for patients receiving radiation therapy [23].
Surgery was suggested to be better than radiotherapy in cases of
extremity ES family tumors with achievable adequate surgical
margins, and thus surgery was the optimal treatment for sites
like extremities, which brought a better prognosis to patients
[20].
Out results show that surgery is superior to SR. Our results are
consistent with those of several previous researchers [7,18-20],
which also indicated that additional radiotherapy did not show
better outcomes when compared with surgery alone. However, the
location of tumor may influence the efficacy of surgery. As
reported previously, surgery was the best treatment for small
tumors at humerus, yet surgery was only recommended for large
tumors when good functional results and quality of life can be
expected, and adequate surgical margins are achievable. The best
treatment is uncertain for long bones that need to be rebuilt
after large segmental resection (femur, tibia, and humerus)
[18]. Moreover, the use of surgery for pelvic tumors in Ewing
sarcoma is controversial [24,25].
Surgery combined with radiotherapy is the standard of care in
the majority of high-risk extremity soft-tissue sarcomas
[26]. Several retrospective studies reported that combined
therapy had a local tumor control advantage over surgery alone,
especially when tumor was larger than 200 mL at diagnosis or the
removal of tissues were incomplete during surgery
[23,27,28]. However, we did not find any survival benefit when
combined intervention was compared with surgery alone. Moreover,
combined radiotherapy after surgery resulted in increased risk
of long-term treatment-associated toxicities [7]. Due to the
lack of sufficient direct data, the adverse effects of SR and
surgery were not compared in our network meta-analysis.
A previous meta-analysis enrolled eight retrospective clinical
trials and reported inconsistent results in the efficacy of
radiotherapy compared with surgery in localized Ewing sarcoma
[1]. Whereas in our analysis, five newer studies were included,
and one article was excluded due to lack of sufficient data
[22]. Moreover, in the present analysis, we focused on the
efficacy of local control strategies after neoadjuvant
chemotherapy. Neoadjuvant chemotherapy helps to treat the
disease early, reducing the chance of metastatic dissemination
and also reduces tumor volume, making it resectable.
Some limitation of this study need to be highlighted. Firstly,
the number of studies enrolled for our analysis were very
limited. We were unable to investigate the effect of different
local control strategies on overall survival, disease-free
survival and survival rate with a shorter follow-up time due to
the lack of sufficient data. Although the network meta-analysis
enlarges source of evidence for different comparisons, we still
need direct evidence for a robust conclusion. Secondly, since
all local control strategies for Ewing sarcoma were performed
after neoadjuvant chemotherapy, we did not specify different
regimens and protocols for chemotherapy. This might confound the
efficacy of local control strategies. Yet all the regimens and
protocols used in enrolled RCTs were standard first-line
treatments, the efficacy of which have been proven in previous
studies. Thirdly, the used local treatment was the
clinicians’choice based on patient and tumor characteristics.
Radiation therapy is often used in cases of narrow or
intralesional surgical margins or poor histological response to
chemotherapy or when surgery would be too mutilating.
Additionally, results of survival analysis were reported by odds
ratios with extracted binary data from original articles. Hence,
we were not able to compare the survival curves of different
local control strategies. Moreover, since no reliable RCTs have
been performed regarding to the efficacy of local control
strategies on Ewing sarcoma patients, we enrolled only
retrospective cohort studies in our analysis. The quality and
reliability of involved data may thus limit the interpretation
of our results.
In conclusion, this network meta-analysis suggested that surgery
might be the optimal option for improving 5-LR and 5-EFSR of
Ewing sarcoma patients. However, due to the lack of high-quality
data, the results should be interpreted with caution. The choice
of local control strategy should be decided through
consideration of patient characteristics, potential adverse
effects, and patient preference. Further research and
well-designed randomized clinical trials are warranted to
clarify the optimal local control strategy for Ewing sarcoma.
Contributors:
HZ,YL,XX: substantial contribution to the conception and design
of the work; HZ,SZ,YX: acquisition, analysis, and interpretation
of the data; HZ: drafting of the manuscript; TF: revising the
manuscript critically. All authors have read and approved the
final article.
Funding:
None; Competing interests: None stated.
|
What This Study Adds? |
Surgery is the optimal option for improving
5-year local recurrence and 5-year event free survival
in Ewing sarcoma patients, following neoadjuvant
chemotherapy.
|
REFERENCES
1. Werier J, Yao X,
Caudrelier JM, Di Primio G, Ghert M, Gupta AA, et al. A
systematic review of optimal treatment strategies for localized
Ewing’s sarcoma of bone after neo-adjuvant chemotherapy. Surg
Oncol. 2016;25:16-23.
2. Bolling T, Hardes J,
Dirksen U. Management of bone tumours in paediatric oncology.
Clin Oncol (R Coll Radiol). 2013;25:19-26.
3. Moore DD, Haydon RC.
Ewing’s sarcoma of bone. Cancer Treat Res. 2014;162:93-115.
4. Falk S, Alpert M.
Five-year survival of patients with Ewing’s sarcoma. Surg
Gynecol Obstet. 1967;124:319-24.
5. Pretz JL, Barysauskas
CM, George S, Hornick JL, Raut CP, Chen YE, et al.
Localized adult ewing sarcoma: Favorable outcomes with
alternating vincristine, doxorubicin, cyclo-phosphamide, and
ifosfamide, etoposide (VDC/IE)-based multimodality therapy.
Oncologist. 2017;22:1265-70.
6. Wardelmann E, Haas R,
Bovée J, Terrier P, Lazar A, Messiou C, et al. Evaluation
of Response After Neoadjuvant Treatment in Soft Tissue Sarcomas;
the European Organization for Research and Treatment of
Cancer–Soft Tissue and Bone Sarcoma Group (EORTC–STBSG)
Recommendations for Pathological Examination and Reporting. Eur
J Cancer. 2016;53:84-95.
7. DuBois SG, Krailo MD,
Gebhardt MC, Donaldson SS, Marcus KJ, Dormans J, et al.
Comparative evaluation of local control strategies in localized
Ewing sarcoma of bone: A report from the Children’s Oncology
Group. Cancer. 2015;121:467-75.
8. Donati D, Yin J, Di
Bella C, Colangeli M, Bacci G, Ferrari S, et al. Local
and distant control in non-metastatic pelvic Ewing’s sarcoma
patients. J Surg Oncol. 2007;96:19-25.
9. Ahmed SK, Randall RL,
DuBois SG, Harmsen WS, Krailo M, Marcus KJ, et al.
Identification of patients with localized Ewing sarcoma at
higher risk for local failure: A report from the Children’s
oncology group. Int J Radiat Oncol Biol Phys. 2017;99:1286-94.
10. Carrie C. Nonmetastatic
Pelvic Ewing Sarcoma: Report of the French Society of Pediatric
Oncology. Med Pediatr Oncol. 1999; 33:444-9;
11. De Marco S, Pollera CF,
Cognetti F. Nephroblastoma in the adult. Med Pediatr Oncol.
1999;33:497-9.
12. Zogopoulos G, Teskey L,
Sung L, Dix D, Grant R, Greenberg ML, et al. Ewing
sarcoma: Favourable results with combined modality therapy and
conservative use of radiotherapy. Pediatr Blood Cancer.
2004;43:35-9.
13. Higgins JP, Altman DG,
Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The
Cochrane Collaboration’s tool for assessing risk of bias in
randomised trials. British Med J. 2011;343:d5928.
14. Slim K, Nini E,
Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological
index for non-randomized studies (minors): Development and
validation of a new instrument. ANZ J Surg. 2003;73:712-6.
15. Ahmed SK, Robinson SI,
Arndt CAS, Petersen IA, Haddock MG, Rose PS, et al.
Pelvis Ewing sarcoma: Local control and survival in the modern
era. Pediatr Blood Cancer. 2017;64:e26504.
16. Laitinen M, Parry M,
Albergo JI, Jeys L, Sumathi V, Grimer R. Outcome of pelvic bone
sarcomas in children. J Pediatr Orthop. 2016;38:537-42.
17. Grevener K, Haveman LM,
Ranft A, van den Berg H, Jung S, Ladenstein R, et al.
Management and outcome of Ewing sarcoma of the head and neck.
Pediatr Blood Cancer. 2016;63:604-10.
18. Bacci G, Palmerini E,
Staals EL, Longhi A, Barbieri E, Alberghini M, et al.
Ewing’s sarcoma family tumors of the humerus: Outcome of
patients treated with radiotherapy, surgery or surgery and
adjuvant radiotherapy. Radiother Oncol. 2009;93:383-7.
19. Yock TI, Krailo M,
Fryer CJ, Donaldson SS, Miser JS, Chen Z, et al. Local
control in pelvic Ewing sarcoma: analysis from INT-0091–A report
from the Children’s Oncology Group. J Clin Oncol.
2006;24:3838-43.
20. Bacci G, Longhi A,
Briccoli A, Bertoni F, Versari M, Picci P. The role of surgical
margins in treatment of Ewing’s sarcoma family tumors:
Experience of a single institution with 512 patients treated
with adjuvant and neoadjuvant chemotherapy. Int J Radiat Oncol
Biol Phys. 2006;65:
766-72.
21. Shankar AG. Local
therapy and other factors Infuencing site of relapse in patients
with localised Ewing’s sarcoma. Eue J Cancer. 1999;35:1698-1704.
22. Sokolov T, Stoyanova A,
Mumdjiev I, Mihova A. Comparison of two treatment approaches to
localized Ewing’s sarcoma. Ortopediya i Travmatologiya. 2000;36:
509-15.
23. Shankar AG, Pinkerton
CR, Atra A, Ashley S, Lewis I, Spooner D, et al. Local
therapy and other factors influencing site of relapse in
patients with localised Ewing’s sarcoma. United Kingdom
Children’s Cancer Study Group (UKCCSG). Eur J Cancer.
1999;35:1698-704.
24. Kawai A, Healey JH,
Boland PJ, Lin PP, Huvos AG, Meyers PA. Prognostic factors for
patients with sarcomas of the pelvic bones. Cancer.
1998;82:851-9.
25. Laitinen M, Parry M,
Albergo JI, Jeys L, Sumathi V, Grimer R. Outcome of pelvic bone
sarcomas in children. J Pediatr Orthop. 2018;38:537-42.
26. O’Sullivan B, Davis AM,
Turcotte R, Bell R, Catton C, Chabot P, et al.
Preoperative versus postoperative radiotherapy in soft-tissue
sarcoma of the limbs: A randomised trial. Lancet.
2002;359:2235-41
27. Foulon S, Brennan B,
Gaspar N, Dirksen U, Jeys L, Cassoni A, et al. Can
postoperative radiotherapy be omitted in localised standard-risk
Ewing sarcoma? An observational study of the Euro-EWING group.
Eur J Cancer. 2016;61:128-36.
28. Bacci G, Forni C, Longhi
A, Ferrari S, Donati D, De Paolis M, et al. Long-term
outcome for patients with non-metastatic Ewing’s sarcoma treated
with adjuvant and neoadjuvant chemotherapies. 402 patients
treated at Rizzoli
between 1972 and 1992. Eur J Cancer. 2004;
40:73-83.

