|
|
|
Indian Pediatr 2013;50: 605-607 |
 |
Rhizomelic Chondrodysplasia Punctata With
Maternal Systemic Lupus Erythromatosus
|
|
Amrita Roy, Pranab De and Swapna Chakraborty
From Department of Pediatric Medicine, Medical
College and Hospitals, 88, College Street, Kolkata, India.
Correspondence to: Dr Amrita Roy, 3B, Shyam
Square East, Kolkata 700 003, West Bengal, India.
Email:
[email protected]
Received: December 27, 2012;
Initial Review: January 28, 2013;
Accepted: January 29, 2013.
|
|
We report Rhizomelic Chondrodysplasia
Punctata (RDCP), a rare, autosomal recessive disorder with rhizomelic
shortening of limbs, congenital cataracts and seizures but without any
biochemical abnormality. The mother of the baby developed Systemic Lupus
Erythromatosus (SLE) with Ro/SSA antibodies 11 months after delivery.
Ro/SSA antibodies may generate calreticulin antibodies causing
characteristic skeletal changes.
Key words: Anti Ro/SSA, Punctate epiphyseal
calcification.
|
The classic form of rhizomelic
chondrodysplasia punctata (RCDP) a rare, autosomal recessive
peroxisomal disorder is characterized by proximal shortening
of the limbs, cataracts, distinct facial appearance, growth
failure, psychomotor retardation and seizures[1]. Common
radiological features are punctate epiphyseal
calcifications, metaphyseal abnormalities, coronal clefts in
vertebral bodies [1]. RCDP is usually lethal with 60% deaths
occurring by age 1 year. [2] The characteristic biochemical
profile has been previously described [3]. Recently,
patients with RCDP phenotype but without abnormal
peroxisomal function have been reported usually secondary to
teratogen exposure or maternal diseases [4]. We report a
neonate with features of RCDP without biochemical
abnormality but whose mother was diagnosed having SLE 2
months prior to delivery.
Case Report
This male baby was the first child of
healthy unrelated Indian Hindu parents born at term by
spontaneous vaginal delivery. His mother and father were 25
and 29 years old, respectively. There was no history of
spontaneous abortions or antenatal teratogen exposure. His
birthweight was 2459 g (10-25th percentile), length was 42.5
cm (<10th percentile), and head circumference was 33 cm
(50th percentile). His upper segment to lower segment ratio
was 1.8:1. He was a disproportionately short infant. He had
proximal shortening of both upper and lower limbs, midfacial
hypoplasia with a depressed nasal bridge. and anteverted
nares with a short neck with nuchal fullness, a
barrel-shaped chest. There were no skin lesions.
Ophthalmological examination showed cataract in both eyes.
A skeletal survey showed rhizomelic
shortening of extremities. Bony stippling was noted in
shoulder, elbow, hip and knee joints with metaphyseal
flaring in humerus and femur (Fig. 1). The
pelvis appeared normal, but the spine exhibited minimal
ossification and coronal clefts of the vertebral bodies.
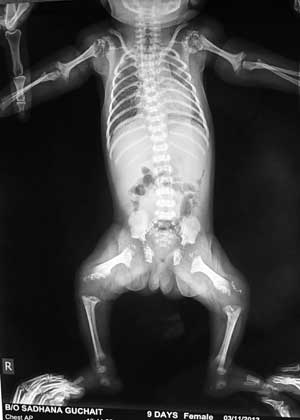 |
|
Fig. 1 Skiagram showing
punctate epiphyseal calcification of shoulder,
elbow, hip and knee joints with metaphyseal flaring
of humerus.
|
Cranial and abdominal ultrasonography and
echocardiography were normal. CT Brain revealed stippled
anterior arch of foramen magnum. A diagnosis of rhizomelic
chondrodysplasia punctata was made. Red blood cell
plasmalogen content was performed as dimethylacetals (DMAs).
The mean levels of C16:0DMA/C16:0 fatty acid, C18:0 DMA/
C18:0 fatty acid, VLCFA and phytanic acid levels were within
the reference range.
Cataract extraction was done. Genetic
assay could not be done due to financial constraints.
Genetic counseling was given to the parents. The infant was
discharged from the nursery at 10 days of age and is
receiving regular physiotherapy.
Two months later, the mother had joint
pain of the hands and the feet and photosensitive malar
rash. Maternal serology results were diagnostic of SLE with
positive antinuclear antibody with a 1:640 titer in a
speckled pattern; positive for extractable nuclear antigen
with an anti-SM level of 42.10 EU/mL [reference:<20 EU/mL]
and anti-RNP level of 192.50 EU/mL [reference: <20.01 EU/mL];
positive for anti- SSA[Ro] 155.4 EU/mL (reference:<25.1 EU/mL).
Other antibodies were negative with normal C4 complement
level. The mother was started on low dose prednisolone 10
mg/day.
Discussion
Chondrodysplasia punctata (CDP) is
characterized by punctuate calcification of cartilage. It
includes peroxisome biogenesis disorders (Zellweger
syndrome, neonatal adrenoleukodystrophy, infantile Refsum
disease, and RCDP Type1), maternal conditions and teratogen
exposure. CDP has four main types, the autosomal dominant (Conradi-Hunermannís
type), autosomal recessive (rhizomelic type), the X-linked
dominant form (Happle) and the X-linked recessive form.
There are three types of RCDP. RCDP Type
1 involves mutations in the PEX7 gene [3]. RCDP Types 2 and
3 are phenotypically similar to RCDP Type 1, but result from
deficiencies of dihydroxyacetone phosphate acyltransferase
and alkyldihydroxyacetone phosphate synthase,
respectively[1].
Though our patient presented with many
characteristic features of RCDP but he differed from other
patients in that there was no abnormality of red blood cell
plasmalogens and phytanic acid levels. Antenatal history of
teratogens like rubella infection, and warfarin or dilantin
use was negative. There are case reports of maternal
autoimmune diseases like SLE and phenylketonuria with CDP in
their babies [5-9]. Our patient is the eleventh reported
RDCP patient born to a mother with SLE. Only 3 have had the
characteristic skin lesions of neonatal lupus erythematosus
(NLE) and none had congenital heart block.
The proposed mechanism for stippling in
CDP-associated maternal lupus is immune mediated by maternal
autoantibodies crossing the placenta in early to
midgestation. These antibodies inhibit a high-affinity
calcium-binding protein of endoplasmic reticulum,
calreticulin. Anti Ro/SSA is an autoantigen complex that may
include calreticulin. Auto-antibodies to calreticulin and
Ro/SSA are involved in the pathogenesis of congenital heart
block and the cutaneous lesions of SLE and may be
responsible for the skeletal changes by inhibiting calcium
binding. Animal model studies showed that immunization of
mice with Ro resulted in the production of anti-Ro, anti-La,
and anti-calreticulin antibodies [10] Our patientís mother
was positive for Ro/SSA. Alternatively maternal
autoantibodies affect the infantís vitamin K metabolism [8]
resulting in bleeding into the epiphyseal cartilage, which
produces the stippled appearance.
Autoantibodies may be the largest single
risk factor for the development of CDP in the neonate but
the presence of autoantibodies cannot be the only
determining factor to predict the occurrence of CDP, because
the incidence of CDP in infants of mothers with SLE is very
low. Management of these babies is mainly supportive.
Cataract extraction and physiotherapy may help. Genetic
counseling is necessary. Monitoring growth and development,
seizure control, vision, hearing, contractures and
orthopedic complications need regular assessment on follow
up.
Contributors: AR and PKD: managed the
patient, reviewed the literature and drafted the manuscript;
PKD will act as guarantor of the study; AR: collected the
data. SP: critically reviewed the article and helped in
drafting the paper. The final manuscript was approved by all
the authors.
Funding: None; Competing interests:
None stated.
References
1. Braverman NE, Moser AB, Steinberg SJ.
Rhizomelic Chondrodysplasia Punctata Type 1. In:
Pagon RA, Bird TD, Dolan CR, Stephens K, editors.
GeneReviews [Internet]. University of Washington, Seattle;
2001 Nov 16. Accessed on 8 April, 2013.
2. Moser A, Moser H, Kreiter N, Raymond
G. Life expectancy in rhizomelic chondrodysplasia punctata.
Am J Hum Genet. 1996;59:99.
3. Phadke SR, Gupta N, Girisha KM, Kabra
M, Maeda M, Vidal E, et al. Rhizomelic
chondrodysplasia punctata type 1: report of mutations in 3
children from India. J Appl Genet. 2010;51:107-10.
4. Shanske AL, Bernstein L, Herzog R.
Chondrodysplasia punctata and maternal autoimmune disease: a
new case and review of the literature. Pediatrics.
2007;120:e436-41.
5. Costa T, Tiller G, Chitayat D,
Silverman E. Maternal systemic lupus erythematosus and
chondrodysplasia punctata in two infants:coincidence or
association? Abstract Book; First meeting of the Bone
Dysplasia Society; June 17-19, 1993.
6. Mansour S, Liberman D, Young I.
Brachytelephalangic chondrodysplasia punctata in an
extremely premature infant. Am J Med Genet.
1994;53:81-2.
7. Kelly TE, Alford BA, Greer KM.
Chondrodysplasia punctata stemming from maternal lupus
erythematosus. Am J Med Genet. 1999;83:397-401.
8. Elcioglu N, Hall CM. Maternal systemic
lupus erythematosus and chondrodysplasia punctata in two
sibs: phenocopy or coincidence? J Med Genet.
1998;35:690Ė4.
9. Toriello HV. Chondrodysplasia punctata
and maternal systemic lupus erythematosus. J Med Genet.
1998;35: 698-9.
10. Suzuki H, Silverman ED, Wu X, Borges C, Zhao S,
Isacovics B, et al. Effect of maternal autoantibodies
on fetal cardiac conduction: an experimental murine model.
Pediatr Res. 2005;57:557-62.
|
|
|
 |
|

