|
|
|
Indian Pediatr 2021;58:631-634 |
 |
Vitamin C Deficiency
and Oxidant Levels in Children With Transfusion-Dependent
b-Thalassemia
|
|
Vasudeva Bhat K, 1 Ratna A
Sharma,1 Sujata M Sharma,1
Priyanka Joshi,2 Bina F
Dias,2 Nikita Shah,1
Maninder Setia,3 Mamta V
Manglani1
From Departments of 1Pediatric Hematology-Oncology, and
2Biochemistry, Lokmanya Tilak Municipal Medical College and General
Hospital, Sion, Mumbai; 3MGM Institute of Health Sciences, Navi Mumbai;
Maharashtra.
Correspondence to: Dr Mamta V Manglani, Director, MCGM- comprehensive
Thalassemia Care, Hematology-Oncology and BMT Centre, CCI Compound,
Borivali (East), Mumbai 400 066, Maharashtra, India.
Email: mmanglani@hotmail.com
Received: May 30, 2020;
Initial review: July 07, 2020;
Accepted: January 01, 2021.
Published online: March 26, 2021;
PII: S097475591600304
|
Objectives: To study vitamin C levels in children
with transfusion-dependent b-thalassemia
and correlate with age, transfusions received and iron overload; and to
study the effect of administering vitamin C on its levels and
Malondialdehyde (MDA) in deficient patients. Methods: This
case-control study enrolled 100 children with transfusion-dependent
b-thalassemia
and 30 healthy controls. MDA levels before and after administration of
vitamin C were performed randomly in 36 children with low vitamin C
levels. Results: 81/95 (85.3%) study subjects vs none in control
group, had low plasma vitamin C levels (P<0.001). Vitamin C
levels were low in 64 of 71 (74.7%) subjects with dietary deficiency,
while none of the 19 (63.3%) controls with dietary deficiency had low
levels (P=0.04). Increasing serum ferritin values correlated with
vitamin C deficiency (P=0.02). The mean level of MDA reduced (P<0.001)
with vitamin C supplementation. Conclusions: Low levels of
vitamin C are common in children with thalassemia. Dietary counseling
along with supplementation with vitamin C, in those with low levels may
prevent oxidative stress.
Keywords: Iron overload, Oxidative stress, Thalassemia,
Scurvy, Supplementation.
|
|
C hildren with
transfusion-dependent b-thalassemia
(TDT) require frequent blood transfusions resulting in iron
overload [1]. Dietary vitamin C is destroyed through
irreversible oxidation by ferric iron deposits, thus leading to
its deficiency causing scurvy [2,3], given the fact that dietary
deficiencies are common in Indian children [4]. However, the
risk of vitamin C supplementation is that excess of vitamin C
enhances iron absorption and also iron-mediated peroxidation of
membrane lipids, causing an increased iron-induced membrane
damage in cultured myocardial cells [2]. This study was thus
designed to assess the plasma levels of vitamin C in Indian
children with TDT and correlate them with various patient and
disease factors, including overload and oxidant levels.
METHODS
This was a cross-sectional study conducted in
the day-care thalassemia centre of a tertiary hospital between
December, 2011 to May, 2012. All children (below 18 years of
age), with TDT and receiving regular transfusions at the center
were included in the study group. Any child who was already
receiving vitamin C prior to enrolment was excluded. In the
control group, 30 asymptomatic children who visited the
pediatric outpatient department were enrolled.
A detailed history including age, gender,
number of transfusions received till date (using record
maintained by the patients), chelation therapy, dietary history
and examination findings (with special reference to signs of
scurvy) were entered in a predesigned proforma after taking
informed consent. All children in the study group underwent the
following investigations: complete blood count with RBC indices,
liver and renal function tests, HIV antibody by ELISA, hepatitis
B surface antigen (HBsAg), anti-HCV antibody, serum ferritin
levels and baseline vitamin C levels prior to transfusion.
Dietary assessment was done by oral questionnaire method by
recalling food eaten in last 48 hours and during weekends [5]
and comparing it with the ICMR food composition tables [6].
Nutritional assessment was done by calculating weight for
height/mid upper arm circumference in less than 5 years, and as
per body mass index in children more than 5 years using the WHO
growth charts [7]. In the control group, a detailed dietary
history with clinical examination was documented in the proforma,
and their blood samples were collected for vitamin C estimation.
All children with low levels of vitamin C
were administered vitamin C orally in therapeutic doses of 200
mg per day for a period of 15 days, while counselling to improve
dietary content of vitamin C was also done. The dose 200 mg was
chosen as non-heme iron absorption enhanced by vitamin C occurs
above this dose [8]. In randomly selected children (n=36)
with low levels of vitamin C, blood was also collected for
oxidant malondialdehyde (MDA) levels prior to administration of
vitamin C and prior to transfusion. Plasma vitamin C and MDA
levels were repeated in these 36 children after completion of 15
days of oral administration of vitamin C.
Vitamin C estimation in plasma was done using
2, 6- dichlorophenol indophenol dye method [9]. A level of
£0.3
mg/dL was considered as deficient according to this method. MDA
estimation was done by modified method of Sadasavidu, et al.
[10].
Sample size was calculated using Stata
Version 15.1 (StataCorp) based on the 64% incidence of vitamin C
deficiency in a previous study [12] of individuals with
thalassemia. With an alpha of 0.05, power of 80%, and delta of
0.14, we estimated the sample size to be 98. Thus, we recruited
100 participants.
Data analyses: Data was entered in MS
Excel (Microsoft Corp.) and converted to Stata Version 10 (Stata
Corp) for analysis. The differences in the categorical outcomes
were tested using the chi square test or Fisher exact test and
the differences in means of the continuous variables were tested
using the t test. We calculated the correlation
coefficient (r) between vitamin C levels, MDA and ferritin
levels. A P value of <0.05 was considered statistically
significant.
RESULTS
A total of 100 children with TDT were
enrolled. Of these, 95 were evaluable of which 61 (64.2%) were
males (median age 9 years, IQR 7-13 years). In control group, of
the 30 children enrolled, 16 (53.3%) were males (median age - 9
years, IQR 7.2-12 years). There was no statistically significant
difference between the age and gender distribution in these two
groups (P=0.56 and 0.29, respectively). The mean (SD)
number of transfusions was 205 (111.5) and serum ferritin level
was 4634.5 (2980.3) ng/mL. There was no statistically
significant difference in the nutritional status between the
study and control group (P=0.4); however, there were
higher percentage of under-nourished children in the study group
(90% vs 64%).
Bone pains (4 children) and gum bleeds (3
children) were seen only in the study group (P=0.69).
Signs of scurvy were seen in 5 (5.3%) (Gum hypertrophy in 2 and
typical skin changes in 3 children) of the children in study
group whereas in none in control group (P=0.45). Eighty
three children (87.4%) were on regular chelation, of which 54
(65.1%) children were on deferasirox, while 29 were receiving
deferiprone (34.9%). Two (2.1%) children with TDT were HIV-1
infected, 18 (19%) were positive for anti-HCV antibodies and
none were HBsAg positive. The mean (SD) value of vitamin C in
study group was 0.2 mg/dL (0.1) and in controls was 0.8 (0.2)
mg/dL (P<0.001).
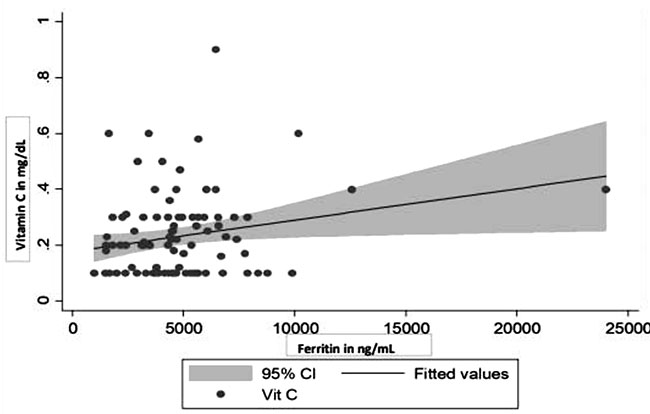 |
|
Fig. 1 Scatter diagram depicting
increasing serum ferritin values significantly
correlated with vitamin C deficiency.
|
Plasma vitamin C levels were low in 81
(85.3%) children in the study group, while all children in
control arm had normal plasma vitamin C levels despite
comparable dietary deficiency of vitamin C (P<0.001).
Table I depicts the correlation of dietary deficiency with
low plasma vitamin C levels in the 2 groups. Age (P=0.86),
number of transfusions received (P=0.67), chelation (P=0.84),
and associated infections (HIV, P=0.55, anti-HCV antibody
positive, P=0.63) did not have any correlation with
vitamin C levels, while increasing serum ferritin values
correlated with vitamin C deficiency (r=0.3, P= 0.02) (Fig.1).
There was a correlation between higher serum ferritin values and
MDA levels done prior to administration of vitamin C (r=0.35,
P=0.03). On administration of vitamin C, the mean (SD)
levels of vitamin C rose from 0.2 ( 0.1) mg/dL to 0.8 ( 0.2) mg/dL
in those with low plasma levels of vitamin C (P<0.001).
The mean (SD) level of MDA dropped from 17.1 ( 7.0) nmol/mL to
8.1 ( 2.5) nmol/mL after 15 days of administration of vitamin C
(P<0.001).
Table I Vitamin C Dietary Deficiency and Plasma Levels in Children With Transfusion-Dependent Thalassemia and Controls
| Dietary |
Study group, |
Control group,a |
| deficiency |
n=95 |
n=30 |
|
Normal level |
Low level |
Total |
Normal level |
|
n=14 |
n=81 |
|
n=30 |
|
(>0.3 mg/dL) |
(£0.3mg/dL) |
|
(>0.3 mg/dL) |
| Present |
7 (50) |
64 (79) |
71 (74.7) |
19 (63.3) |
| Absent |
7 (50) |
17 (21) |
24 (25.3) |
11 (36.7) |
| All values in no. (%).
aNone had low vitamin C level. P=0.04 for low vitamin C
levels in diet deficient children in study and control
group. |
DISCUSSION
In addition to occasional case reports of
scurvy occurring in children with thalassemia [3], a few studies
have also described vitamin C deficiency in these children
[11,12]. We determined the magnitude of vitamin C deficiency in
Indian children with TDT and its impact on oxidant (MDA) levels.
Clinical symptoms and/or signs of scurvy were seen in 7% of
patients in the study group and none in the control group, and
vitamin C deficiency was associated with iron overload and
higher oxidant (MDA) levels.
Previous studies from various countries have
reported vitamin C deficiency in 64-100% of patients with
thalassemia [11-13], similar to 85.3% reported in this study.
Hussien, et al. [12] reported suboptimal plasma levels of
vitamin C in all children with TDT, despite a diet sufficient in
vitamin C. We also found low levels of vitamin C in 70.8% of
children with TDT without dietary deficiency, though it was
higher in those with dietary deficiency (90.1%). In the control
group, irrespective of dietary deficiency, all children had
normal vitamin C levels, probably due to lower or no oxidant
stress in them. Similar to our findings, a relation between iron
overload and vitamin C deficiency has also been reported by
Hussien, et al. [12].
The levels of oxidants and lipid peroxides
are high in children with TDT due to the accumulation of free
iron radicals and production of reactive oxygen species. A MDA
higher level signifies peroxidative damage to lipid mem-branes
in children with TDT [14,15]. Our results are similar to other
studies done in patients with transfusion-dependent
b-thalassemia,
which have also found a marked imbalance in the oxidant and
antioxidant status with reduction in the antioxidants and
increase in the oxidant level with vitamin C deficiency [14,16];
although, few authors have not reported such an association
[15].
A significant reduction in the MDA levels
oxidant load was observed after administration of vitamin C,
suggesting higher oxidative stress in children with vitamin C
deficiency. This also confirmed that supplementation of vitamin
C does not further increase the oxidative stress and hence is
safe to be given in children who are deficient.
The present study had some limitations. Only
plasma vitamin C and MDA levels were measured out of numerous
antioxidants and oxidants that are present in the body. Iron
overload was estimated using serum ferritin alone which may also
be elevated due to infections and inflammation. Tissue iron
overload was not estimated using T2*weighted magnetic resonance
imaging.
Besides regular packed red cells and adequate
iron chelation, maintaining vitamin C homeostasis is the key to
reducing the oxidative stress, thereby protecting these children
from myocardial damage and consequent mortality. Despite the
fact that there was no statistically significant difference in
nutritional status between the two groups, the proportion of
undernourished children with TDT was higher; hence, improved
dietary intake through counseling and supplementing vitamin C in
those children with TDT with low plasma vitamin C levels, will
improve outcomes in these children.
Ethics clearance: Institutional Ethics
Committee of LTMM College and LTMG Hospital, Mumbai; No.
PS/IECHR/DISS/105(11/10).
Contributors: VB: conducted the study and
prepared the draft of the manuscript; RS: helped in management
of the patients, monitored the outcomes and helped in analysing
the results; SS: helped in collecting data and managing the
patients; PJ: performed the tests in the laboratory; BD:
supervised the laboratory testing of the samples and helped in
correlating with the clinical findings; MS: helped in planning
the study and did the statistical analysis; NS: helped in
revising the draft; MM: conceptualized the study, guided
throughout the study and finalized the draft. All authors
approved the final version of manu- script, and are accountable
for all aspects related to the study.
Funding: Intramural funds; Competing
interests: None stated.
|
WHAT THIS STUDY ADDS?
• Children with transfusion-dependent thalassemia are
deficient in vitamin C and are more likely to develop
scurvy, besides posing a risk of oxidative stress.
|
REFERENCES
1. Verma IC, Saxena R, Kohli S. Past, present
and future scenario of thalassaemic care and control in India.
Indian J Med Res. 2011;134:507-21.
2. Darvishi KH, Emami ZA, Sharifi H, Jalali
H. Is Vitamin C supplementation in patients with
b-thalassemia
major beneficial or detrimental? Hemoglobin. 2016;40:293-4.
3. Munni R, Mawaha R, Sethuraman G, Trehan A.
Scurvey in transfusion dependent betathalassemia. Indian Pediatr.
1999:504-6.
4. Srihari G, Eilander A, Muthayya S, Kurpad
AV, Seshadri S. Nutritional status of affluent Indian school
children: What and how much do we know? Indian Pediatr.
2007:199-203.
5. Subar AF, Thompson FE, Kipnis V, et al.
Comparative validation of the Block, Willett, and National
Cancer Institute food frequency questionnaires: the Eating at
America’s Table Study. Am J Epidemiol. 2001;154: 1089-99.
6. Longvah T, Ananthan R, Bhaskarachary K, et
al. Indian food composition tables. National Institute of
Nutrition. Indian Council of Medical Research,2017.
7. WHO. The WHO Child Growth Standards.
Accessed on October 9, 2020. Available from:
https://www.who.int/childgrowth/en/
8. Kliegman R, Stanton B, St. Geme JW, Schor,
NF et al. (2016). Nelson textbook of pediatrics (Edition 20.).
Elsevier. p. 324.
9. VanderJagt DJ, Garry PJ, Hunt WC.
Ascorbate in plasma as measured by liquid chromatography and by
dichloropheno-lindophenol colorimetry. Clin Chem.
1986;32:1004-6.
10. Sasikala M, Subramanyam C, Sadasivudu B.
Early oxidative change in low density lipoproteins during
progressive chronic renal failure. Ind J Clin Biochem.
1999;14:176.
11. Chapman RW, Hussain MA, Gorman A, et al.
Effect of ascorbic acid deficiency on serum ferritin
concentration in patients with beta-thalassaemia major and iron
overload. J Clin Pathol. 1982;35:487-91.
12. Hussien JN A-KS, Al-Zuhairy SH. Role of
vitamin C supplementation on iron overload and oxidative stress
in beta thalassemia major patients in Maysan Province-Iraq. Kufa
Medical J. 2017;17:111-27.
13. Wapnick AA LS, Krawitz P, Seftel HC,
Charlton RW, Bothwell TH. Effects of iron overload on ascorbic
acid metabolism. BMJ. 1968;3:704-7.
14. Naithani R, Chandra J, Bhattacharjee J,
Verma P, Narayan S. Peroxidative stress and antioxidant enzymes
in children with b-thalassemia
major. Pediatr Blood Cancer. 2006; 46:780-5.
15. Gunarsih A, Amalia P, Boediman I.
Variables associated with malondialdehyde level in thalassemia
major patients. Paediatr Indone. 2012;52:125-31.
16. Ghone RA, Kumbar KM, Suryakar AN, Katkam RV, Joshi NG.
Oxidative stress and disturbance in antioxidant balance in beta
thalassemia major. Ind J Clin Biochem. 2008; 23: 337-40.
|
|
|
 |
|

