|
|
|
Indian Pediatr 2021;58: 624-630 |
 |
Epinephrine Plus Vasopressin vs
Epinephrine Plus Placebo in Pediatric Intensive Care Unit
Cardiopulmonary Resuscitation: A Randomized Double Blind
Controlled Clinical Trial
|
|
Abraar Sheriff, Ramachandran Rameshkumar, Muthu Chidambaram, Kaushik
Maulik, Routhu Santhosh Kumar, Atahar Jamal, Rohit Bhowmick, Niranjan
Biswal, Subramanian Mahadevan
From Division of Pediatric Critical Care, Department of Pediatrics,
Jawaharlal Institute of Postgraduate Medical Education and Research
(JIPMER), Puducherry.
Correspondence to: Dr Rameshkumar R, Associate Professor,
Division of Pediatric Critical care, Department of Pediatrics,
Jawaharlal Institute of Postgraduate Medical Education and Research
(JIPMER), Puducherry 605 006. Email:
krramesh_iway@yahoo.co.in
Received: June 05, 2020;
Initial review: September 16, 2020;
Accepted: February 09, 2021.
Trial Registration: CTRI/2019/01/017200.
Published online: February 19, 2021;
PII: S097475591600287
|
|
Objective: To compare
the efficacy of epinephrine plus vasopressin vs epinephrine plus
placebo in the pediatric intensive care unit (PICU)
cardiopulmonary resuscitation (CPR).
Design: Randomized,
double-blind controlled clinical trial.
Setting: PICU in a
tertiary care institute from February, 2019 to May, 2020.
Participants:
Children aged one month to 13 years who required CPR during PICU
stay. Patients in whom vascular access was not available or
return of spontaneous circulation (ROSC) was achieved by
defibrillation without epinephrine were excluded.
Intervention:
Patients were randomized to receive vasopressin 0.1 mL per kg
(=0.8 unit per kg) or placebo (0.1 mL per kg normal saline) in
addition to epinephrine (1:10000) 0.1 mL per kg. The drugs were
given as bolus doses every three minutes until the ROSC or up to
a maximum of five doses, whichever was earlier.
Outcome Measure: The
primary outcome was the proportion of patients who achieved
ROSC. The secondary outcomes were survival rate and functional
status (at 24-hour, PICU, hospital, and 90-day post-discharge),
need for organ supports, length of stay (PICU and hospital), and
adverse effect(s) of the study drugs.
Results: 90 patients
(epinephrine plus vasopressin group, n=45 and epinephrine
plus placebo group, n=45) were analyzed on
intention-to-treat basis. There was no significant difference in
the primary outcome between epinephrine plus vasopressin (n=25,
55.5%) and epinephrine plus placebo groups (n=24, 53.3%)
(Relative risk 1.04, 95% CI 0.71 to 1.52). There was no
significant difference in survival rate at 24-hour (n=7,
15.6% vs. n=8, 17.8%), at PICU, hospital, and 90-day
post-discharge (n=1, 2.2% vs n=1, 2.2%). There was
no difference in other secondary outcomes. No trial drug-related
serious adverse events were observed.
Conclusion: A
combination of epinephrine plus vasopressin did not improve the
rate of return of spontaneous circulation in the pediatric
intensive care unit cardiopulmonary resuscitation as compared
with epinephrine plus placebo.
Keywords: In-hospital cardiac
arrest.
|
C
ardiac arrest is a
dreadful event in pediatric
intensive care unit (PICU). Cardiopulmonary resuscitation
(CPR) involves chest compression and manual ventilation at appropriate intervals.
Return of spontaneous circulation (ROSC) is the initial
therapeutic goal in cardiac arrest and is a measure of
initial success. Vasopressor medications are often used
during CPR. These medications increase aortic diastolic
pressure, thereby improving coronary perfusion pressure,
which facilitates ROSC [1]. Epinephrine is the most widely
studied and the first line vasoactive drug as per the
pediatric advanced life support (PALS) guidelines [2].
Vasopressin, a potent vasoconstrictor, is well studied in
adult cardiac arrest [3]. The recent advanced
cardio-vascular life support guideline recommends that
Vaso-pressin, combined with epinephrine, may be considered
in adult cardiac arrest resuscitation [3]. Though the
pediatric in-hospital cardiac arrest (IHCA) outcome has
improved from 39% to 77% in high-income countries, data from
low-and middle-income countries are lacking or are
under-reported [2]. Animal studies, case series, and
feasibility pilot studies have shown encouraging results for
the use of vasopressin in pediatric cardiac arrest [4-6].
This study hypothesized that epinephrine plus vasopressin
would be associated with a higher rate of ROSC as compared
to epinephrine plus placebo in the pediatric intensive care
unit cardio-pulmonary resuscitation.
METHODS
This randomized, double-blind controlled
clinical trial was undertaken in PICU of a tertiary care
academic hospital from February, 2019 to May, 2020. Ours is
a 19 bedded level-III PICU, receiving critically ill
children 24 hours a day throughout the year. Though our PICU
is a predominantly medical ICU, it also receives complicated
surgical and trauma patients. The PICU has facilities for
providing multimodal hemodynamic and neuromonito-ring,
mechanical ventilation, and high-frequency venti-lation. It
is also equipped with an in-house blood gas analyzer with a
co-oximeter module, osmometer, thera-peutic plasma exchange,
and renal replacement therapy. During the study period, the
baseline mortality in our PICU was 20%, and the average
length of PICU stay was six days, with a bed occupancy rate
of 80%.
The trial was approved, and its progress
was reviewed yearly by the institute ethics committee.
Written informed consent was obtained from the
parent/legally authorized representatives of all patients
getting admitted to PICU at the time of transfer-in, stating
that their child might be enrolled in the study if the child
required CPR during the PICU stay. Children aged one month
to 13 years, admitted in PICU, and who required CPR during
their PICU stay were enrolled. Children who had a cardiac
arrest outside of PICU and were shifted to PICU for
post-cardiac arrest care were not enrolled. Children with
either of the following conditions were also excluded (i)
patients in whom vascular access was not available (ii)
ROSC was achieved by defibrillation without the requirement
of Epinephrine.
A computer-generated, unstratified, block
randomi-zation with variable block sizes of four, six, and
eight was used with an allocation ratio of 1:1 by a person
not involved in the study. Individual assignments were kept
in serially numbered boxes. Each box contained ten
identically looking one mL ampoules of either vasopressin or
placebo (normal saline). The original label in each ampoule
was removed and replaced by an opaque paper. Each box was
serially numbered and allocated to the patient according to
the random sequence. The serially-numbered trial drug boxes
were kept in a separate place in PICU to avoid the wrong
allocation in the stressful environment. Only one trial drug
box was kept in the crash cart, which contained all the
emergency drugs and equipment required for CPR. The nurses
were instructed to open the trial drug box, which was kept
in the crash cart during CPR. The investigator ensured the
replacement of the trial drug box in the crash cart
according to the serial number once the trial drug was used.
Multiple simulation sessions were carried out and discussed
before the start of the study. Injection normal saline
(sodium chloride 0.9%, 1 mL, Serum Institute of India Pvt
Ltd), injection epinephrine (Bioaderna, 1 mg per 1 mL,
Health Biotech Ltd) and injection vasopressin (Vascel
20, 20 Unit per mL, CELON laboratories
Pvt Ltd) were used in this study. The institute’s central
pharmacy supplied the trial drugs. The participants,
treating team and nurses administering the medications, and
the investigators, were unaware of the treatment
assignments. The person who collected and entered the data
into the datasheet and the study statistician were unaware
of the treatment assign-ment throughout the analyses. The
treatment assignment was disclosed, after the first draft of
the result was finalized.
All patients received CPR in accordance
with the PALS-2015 guidelines established by the American
Heart Association (AHA) [2]. This includes the support of
airway, breathing, including supplemental oxygen, evaluation
of cardiac rhythm, high-quality CPR with minimally
interrupted chest compressions, electrical defibrillation if
appropriate, and medications except for the trial drugs. The
resuscitation team members were trained to provide CPR as
per the PALS 2015 guidelines [2]. The facility for standby
extracorporeal membrane oxygenation (ECMO) is not available
in the study site (PICU). Our hospital has no approved
guidelines for ‘Do not resuscitate’ instructions.
Epinephrine plus vasopressin group received intravenous
epinephrine (1:10000) 0.1 mL per kg and vasopressin (1:1.5
dilution in normal saline) 0.1 mL per kg (=0.8 unit per kg;
maximum dose of 5 mL, 40 unit). Epinephrine plus placebo
group received intravenous epinephrine (1:10000) 0.1 mL per
kg and placebo (1:1.5 dilution in normal saline) 0.1 mL per
kg. The trial drugs were given as bolus doses, concurrently
if two vascular accesses were available or within 10 seconds
gap if one vascular access was available. The trial drugs
were given at an interval of every three minutes until ROSC
or a maximum of five doses, whichever was earlier. Three mL
normal saline flush was given after adminis-tration of each
dose of the trial drug. Subsequently, if needed, epinephrine
was continued as per protocol. Post-resuscitation care was
provided to the patients who achieved ROSC as per the unit
protocol (from PALS-2015 guidelines) [2]. All patients were
followed up until death or 90 day post-discharge. The
functional status of the survivor was assessed by using the
pediatric cerebral performance category (PCPC) scale and
pediatric overall performance category (POPC) scale (lower
the score, better the neurological outcome) [7]. Data
regarding the cardiac arrest events and their outcomes were
collected as per the Utstein style template and in
the predesigned proforma [8-10].
The primary outcome was the proportion of
patients who achieved ROSC. The secondary outcomes were (i)
survival rate (at 24 hours, PICU, hospital, and 90-day of
discharge), (ii) functional status (at PICU,
hospital, and 90-day of discharge), (iii) need for
organ support(s), (iv) length of stay in PICU and
hospital, and (v) adverse effect(s) of the study
drugs if any. ROSC was defined as the restoration of a
spontaneous perfusing rhythm that results in more than an
occasional gasp, fleeting palpable pulse, or arterial
waveform [2,3,10]. Sustained ROSC was defined as not
requiring chest compressions for 20 consecutive minutes
after obtaining ROSC and signs of perfusion [2,3,10]. The
probability of adverse trial drug reaction was assessed by
Naranjo algorithm [11].
The ROSC rate varies between 47% and
64.6%, as reported by previous studies [12,13]. We assumed
that the primary outcome of interest in the control group
was 50%. We calculated the sample size based upon the
assumption of 30% improvement in the primary outcome by the
intervention with 80% power at the 5% significance
(two-sided) and 1:1 allocation. Thirty-nine patients were
required in each group by calculation. With a 10% attrition
rate, the final sample size was estimated as 86 [12-14]. The
sample size was calculated using the software nQuery version
4.0.
Statistical analysis: Data were
analyzed according to their assigned groups (intention to
treat analysis). The distribution of data was checked with
the Kolmogorov-Smirnov Z test. Continuous variables were
compared between the two groups by Student’s t-test
for normally distributed or by the Mann-Whitney U
test for skewed data. Proportions were compared by the
Chi-square test (or Fisher’s exact test if expected cell
frequencies were less than five). Kaplan-Meier curve and
log-rank test were used to analyze ‘time to event’ data
followed by Cox proportional hazard regression analysis to
adjust for the prespecified baseline factors (age, sex, and
PRISM-III score). The relative risk and hazard ratio, with a
95% confidence interval, was calculated as appropriate. All
tests were two-tailed, and a P value of less than
0.05 was considered statistically significant. IBM SPSS
software 20.0 (IBM Corp) and Epi Info 7 (7.0.9.7, CDC) were
used for data analysis.
RESULTS
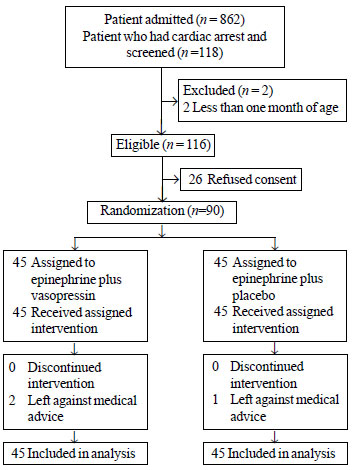
The study flow is depicted in Fig.
1. Ninety patients were enrolled (epinephrine plus
vasopressin, n=45, and epinephrine plus placebo n=45)
after the screening of 118 patients. The baseline
characteristics and clinical variables are described in
Table I. The median (IQR) time to first cardiac arrest
since admission was similar between groups [2 (1-7) vs 2
(1-5) day; P=0.75]. The most common (80%) arrest
rhythm was pulseless electrical activity (PEA). Hemodynamic
abnormality (67.8%) was the most common event that led to
arrest, followed by respiratory events (23.3%). Respiratory
failure was an underlying illness in 76 (84.4%) patients and
sepsis in 60 (66.7%) patients. The median (IQR) duration of
CPR was similar between groups [18 (10-30) vs 15 (6–30)
minutes; P=0.96].
 |
|
Fig. 1 Study flow chart.
|
Table I Baseline Characteristics and Clinical Variables of the Two Study Groups
| Variables |
Epinephrine plus |
Epinephrine plus |
|
vasopressin group |
placebo group |
|
(n = 45) |
(n = 45) |
|
Age, ya |
2.5 (3.3) |
3 (4.4) |
| Male: female |
25:20 |
28:17 |
|
Body mass indexa |
– 2 (1.9) |
– 1.9 (2.0) |
|
Pediatric risk of mortality - III scorea |
19.6 (9.6) |
18 (8.6) |
| Arrest rhythm |
|
|
| Pulseless electrical
activity |
38 (84.5) |
34 (75.6) |
| Asystole |
6 (13.3) |
10 (22.2) |
| Pulseless ventricular
tachycardia |
1 (2.2) |
1 (2.2) |
| Events leading to arrest
|
|
|
| Hemodynamic abnormality
|
31 (68.9) |
30 (66.7) |
| Respiratory events |
11 (24.4) |
10 (22.2) |
| Rhythm disturbance |
3 (6.7) |
5 (11.1) |
| Illness category |
|
|
| Medical condition |
40 (89) |
42 (93.3) |
| Surgical condition |
5 (11) |
3 (6.7) |
|
Diagnosis and underlying illnessb
|
|
|
| Respiratory failure |
38 (84.4) |
38 (84.4) |
|
Sepsis and shockd |
37 (82.2) |
23 (51.1) |
| CNS illness |
19 (42.2) |
22 (49) |
| Pneumonia |
24 (53.3) |
17 (37.8) |
| Congenital heart disease
|
7 (15.6) |
10 (22.2) |
| Renal insufficiency |
21 (46.7) |
16 (35.6) |
| Hepatic insufficiency |
21 (46.7) |
14 (31.1) |
| Malignancy |
5 (11) |
4 (9) |
|
Intervention in place at the time of eventc
|
|
|
| Mechanical ventilation |
44 (97.8) |
43 (95.6) |
| EtCO2 monitoring |
44 (97.8) |
43 (95.6) |
| Arterial line |
37 (82.2) |
34 (75.6) |
| Central venous access |
43 (95.6) |
42 (93.3) |
| Vasoactive drug infusion
|
40 (89) |
39 (86.7) |
| Renal replacement therapy |
8 (17.8) |
6 (13.3) |
| Intervention done during
CPR |
|
|
| Sodium bicarbonate |
14 (31.1) |
23 (51.1) |
|
Calcium gluconate
|
8 (17.8) |
14 (31.1) |
| Atropine |
1 (2.2) |
2 (4.4) |
| Defibrillation |
1 (2.2) |
1 (2.2) |
|
Doses of study druga |
3.6 (1.6) |
3.5 (1.6) |
| Data in no. (%)
or amean (SD). CNS: central nervous system; SD:
stan-dard deviation; EtCO2: end-tidal carbon
dioxide; CPR: cardio-pulmonary resuscitation;
bPatient had one or more conditions; chad one or
more interventions. Hence, the cumulative totals do
not necessarily equal. Three patients also received
EtCO2 monitoring after placement of endotracheal
tube during CPR; dP=0.002. |
The proportion of patients who achieved
ROSC was similar in epinephrine plus vasopressin group and
epinephrine plus placebo group [RR (95% CI) 1.04
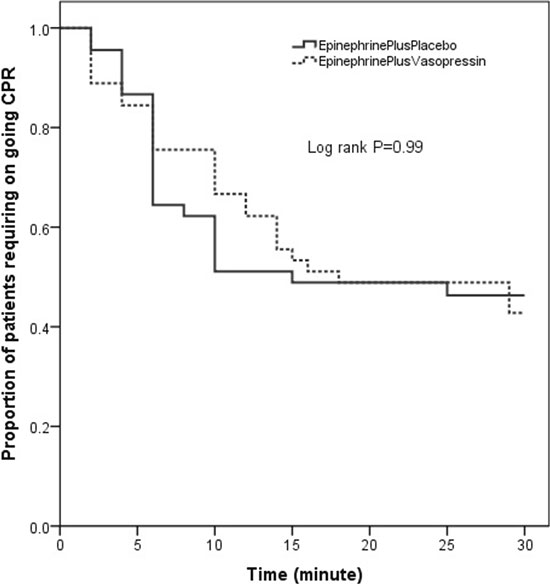
(0.71-1.52); P=0.83]. The time to achieve ROSC and
the proportion of patients requiring ongoing CPR was similar
between two groups during the first 30 minutes of CPR [Log
rank P=0.99] (Fig. 2). Among ROSC achieved
patients (n=49), the median (IQR) time taken to ROSC
was similar between two groups [10 (4-14) vs 6 (5-10)
minutes, P=0.21]. The proportion of patients who
underwent CPR beyond 30 minutes was also similar between two
groups [RR (95% CI) 0.50 (0.19-1.35); P=0.16] and
none achieved ROSC. There was no significant difference in
the proportion of patients who achieved sustained ROSC in
the study groups [44.4% vs 53.3%; P=0.40]. The
survival to hospital discharge was similar in both groups [n=1
each]. Mean (SD) diastolic blood pressure (DBP) was similar
in epinephrine plus vasopressin group as compared to
epinephrine plus placebo group during CPR (38.1 (11.5) mm Hg
vs 37.1 (13.4) mm Hg, P=0.77). There was no
significant difference in the other secondary outcomes
between study groups (Table II). In epinephrine plus
vasopressin group, one patient developed pulseless
ventricular tachycardia which converted into asystole during
the third cycle of CPR. There were no serious trial
drug-related adverse events observed.
 |
|
Fig. 2 Kaplan Meier curves showing time to
return of spontaneous circulation (ROSC) and the
proportion of patients requiring on-going cardio
pulmonary resuscitation (CPR) between the two study
groups.
|
Table II Primary and Secondary Outcomes of the Study Groups
| Variables |
Epinephrine plus |
Epinephrine plus |
Relative risk |
P value |
|
vasopressin group |
placebo group |
(95% CI) |
|
|
(n = 45) |
(n =45) |
|
|
| Primary outcome |
| Proportion of patients
achieved ROSC |
25 (55.5) |
24 (53.3) |
1.04 (0.71-1.52) |
0.83a |
| Proportion of patients
achieved sustained ROSC |
20 (44.4) |
24 (53.3) |
0.83 (0.54-1.28) |
0.40a |
| Secondary
outcomes |
| Survival rate at 24 – hour |
7 (15.6) |
8 (17.8) |
0.88 (0.35-2.21) |
0.78a |
| At PICU discharge |
1 (2.2) |
1 (2.2) |
1.00 (0.06-15.50) |
1.00c |
| At Hospital discharge |
1 (2.2) |
1 (2.2) |
1.00 (0.06-15.50) |
1.00c |
| At 90-day post-discharge |
1 (2.2) |
1 (2.2) |
1.00 (0.06-15.50) |
1.00c |
| Functional
status |
| PCPC score – 1 (mild) |
- |
1 (2.2) |
- |
- |
| POPC score – 4 (severe) |
1 (2.2) |
- |
- |
- |
|
Organ support therapy among patients achieved
ROSCa,b |
| Mechanical ventilation, h
|
1.5 (0.5-12) |
5 (1.3-33) |
- |
0.07b |
| Vasoactive therapy, h |
1.5 (0.4-8) |
4 (1.3-19) |
- |
0.07b |
|
RRT, hc |
1 (1-14.3) |
50 (1-137) |
- |
0.13b |
| PICU stay, h |
1.5 (0.5-12) |
5 (1.3-33) |
- |
0.08b |
| Hospital stay, h |
1.5 (0.5-12) |
5 (1.3-33) |
- |
0.08b |
|
Data are presented as no.(%)
except amedian (IQR). ROSC: return of spontaneous
circulation; CI: confidence interval; IQR:
interquartile range; RRT: renal replacement therapy;
PCPC: pediatric cerebral performance category; POPC:
pediatric overall performance category. b25 in
epinephrine plus vasopressin group and 24 in
epinephrine plus placebo group; cseven in
epinephrine plus vasopressin and six in epinephrine
plus placebo group received RRT support after ROSC.
|
DISCUSSION
This randomized controlled trial enrolled
90 patients who underwent CPR in PICU. We found no
significant difference in the proportion of patients who
achieved ROSC and survival rate between the Epinephrine plus
Vasopressin group and Epinephrine plus Placebo group. In our
study, the overall rate of achieving ROSC was 54.4%, and
survival to hospital discharge was 2.2%. This observation
contrasts with the data from high-income countries, where
the rates were more than 80% and 40%, respectively [15]. The
potential reasons could be the uniform reporting registries,
universal healthcare prog-rams, training of health care
workers, and accessibility to extracorporeal membrane
oxygenation (ECMO).
The outcome of a pediatric cardiac arrest
depends upon many factors, including the initial presenting
rhythm, the place of cardiac arrest, early recognition of
the arrest, and the underlying conditions. In the previous
studies done in high-income countries, asystole (55%) was
the initial arrest rhythm, and respiratory failure was the
common precipitating factor [1,9]. Nevertheless, they
enrolled patients not only from PICU but also from the
emergency department and general ward [1,9]. In contrast,
this study enrolled patients only from PICU, where stringent
monitoring helped identify the arrest much earlier, before
progressing to asystole. Similar to our study setting,
Rathore, et al. [12] reported bradycardia (52.2%) and sepsis
(71%) as the initial arrest rhythm and underlying diagnosis,
respectively. They reported a higher ROSC rate (64.6%) and
survival to hospital discharge (14%). However, only 21% of
CPR occurred in PICU in that study. In our study, patients
were enrolled only from PICU. So, the study population was
different. Generally, PICU patients are sicker and the
majority of them have multiple organ dysfunction requiring
organ support. Also, the initial rhythm is an important
factor in predicting the outcome; bradycardia rhythm with a
pulse is more likely to recover than pulseless non-shockable
rhythms [12].
At present, only a limited number of
vasopressors are available for use in pediatric CPR, and
insufficient data supporting their use [2]. The pediatric
guidelines were extrapolated from adult clinical trials and
animal studies. Vasopressin acts via the V-1 receptor in the
arterial wall and increases the aortic diastolic pressure,
thereby improving coronary perfusion pressure. In contrast
to epinephrine, there are no
b1
mediated chronotropic and inotropic
actions; hence it enhances the myocardial oxygen delivery
and reduces the myocardial oxygen consumption during CPR and
in the post-resuscitation period [1,16]. Another advantage
of vasopressin includes the continuation of vasoconstrictive
effects, even in severe acidosis, accom-panying cardiac
arrest. Hence, vasopressin can act as a better vasopressor
during CPR, particularly in patients with sepsis-associated
myocardial dysfunction and severe acidosis [16]. However,
vasopressin has a longer duration of action than
epinephrine, where the persistent vaso-constriction may
worsen the myocardial dysfunction in the immediate
post-resuscitation period. Post cardiac arrest myocardial
dysfunction can be caused by various factors, including the
underlying pre-arrest cardiac status, duration and quality
of CPR, and the presence of other organ dysfunction(s). So,
it is difficult to establish the causal relationship between
post-cardiac arrest myocardial dysfunction and vasopressin
use. However, no probable serious adverse event due to the
trial drug was observed in this study.
The feasibility pilot study in pediatric
cardiac arrest by Carroll, et al. [6] reported no
significant difference in ROSC, survival to hospital
discharge, and neurological outcome at discharge between
vasopressin and control groups (who did not receive
vasopressin). Nevertheless, they reported a higher survival
rate at 24 hours in the vasopressin group. Their study was
limited by non-randomization, small sample size, and
addition of vasopressin only after non-response to
epinephrine.
Similarly, Duncan, et al. [1] explored
the use of vasopressin in pediatric in-hospital arrest from
the American Heart Association National Registry of CPR data
[1]. Patients who received vasopressin had a longer median
arrest duration as compared to those who did not. They also
noted that, on multivariate analysis, those who received
vasopressin had a reduced ROSC; however, there was no
difference in survival at 24 hours. Vasopressin was used as
a "drug of last resort" for many of their patients [1], in
contrast to our study, where it was used from the time CPR
was initiated.
In comparison with an adult, children
often present with a non-shockable rhythm, which requires
high-quality chest compressions [15]. A systematic review
that included 26 RCTs and 21704 participants found that
vasopressin did not improve the ROSC rate but improved the
survival to hospital admission compared to epinephrine [17].
However, the combination of epinephrine and vasopressin did
not show any significant outcome benefits as compared to
epinephrine alone [17]. However, most of the included
studies were conducted over two decades back. Hence, these
findings may not reflect the current practice in the growing
era of extracorporeal life support (ECLS) availability.
Though all healthcare providers in our
study have been trained in CPR, the intra- and
inter-personal variations in chest compression were not
monitored objectively. The temporal profiles of end-tidal
carbon dioxide and DBP were not analyzed with the outcome of
the study. Though our study found similar DBP in both the
groups, the pediatric-specific target DBP during CPR is yet
to be studied. However, evidence suggests that those who
achieve DBP of 25 to 30 mm Hg during CPR have a higher
chance of ROSC and survival [15]. Hence, goal-directed CPR
targeting the end-tidal carbon dioxide and DBP needs to be
considered in future study design. The availability of ECMO
service during CPR or after achieving ROSC could have
improved the survival to discharge. Recent studies showed
that extracorporeal CPR (E-CPR) in pediatric cardiac arrest
was associated with shorter resuscitation time and higher
survival rate, ranging from 33-64% [18-20]. The AHA
recommends considering E-CPR during in-hospital pediatric
cardiac arrest, when standard resuscitation has failed,
especially in a potentially reversible cause of cardiac
arrest [2].
The study concludes that a combination of
epinephrine and vasopressin did not improve the rate of
return of spontaneous circulation, survival, and favorable
neuro-logical outcomes in pediatric intensive care unit
cardiac arrest resuscitation as compared to epinephrine and
placebo.
Acknowledgments: Mrs. S. Raja Deepa
B.Com, MCA (JIPMER Campus, Puducherry, India) for blinded
data handling, review and editing of the manuscript; Mr.
Rakesh Mohindra (Punjab University, Chandigarh, India) and
Mrs. Thenmozhi M (M.Sc, Ph.D., Senior Demonstrator, CMC,
Vellore, India) for helping with statistical analysis and
Mrs. Harpreet Kaur (Punjab University, Chandigarh, India),
and Mrs. Neelima Chadha (Tulsi Das Library, PGIMER,
Chandigarh, India) for helping with the medical literature
search.
Note: The preliminary study data was
presented in the 21st National Conference of IAP Intensive
Care Chapter (NCPIC 2019), from 5th to 8th December, 2019,
Bengaluru.
Contributors: RR: had full access to
all the data in the study and took responsibility for the
integrity of the data and the accuracy of the data analysis;
RR: Study concept and design; AS, MC, KM, RSK, AJ, RB:
acquisition, analysis, or interpretation of data and
drafting of the first manuscript; MC, RB, NB, SM: protocol
development and revision of the manuscript; RR, SM: critical
revision of the manuscript for important intellectual
content; RR, NB: study supervision. RR: is the guarantor of
the paper. All authors approved the final version of the
manuscript.
Funding: None; Competing interest:
None stated.
|
WHAT IS ALREADY KNOWN?
• Few
studies have shown promising results of vasopressin
use in pediatric in-hospital cardiopulmonary
resuscitation.
WHAT THIS STUDY ADDS?
•
The combination of
cpinephrine and vasopressin did not improve the rate
of return of spontaneous circulation, survival, and
favorable neurological outcome as compared to
Epinephrine alone.
|
REFERENCES
1. Duncan JM, Meaney P, Simpson P, et al.
Vasopressin for in-hospital pediatric cardiac arrest:
results from the American Heart Association National
Registry of Cardiopulmonary Resuscitation. Pediatr Crit Care
Med. 2009;10: 191-5.
2. de Caen AR, Berg MD, Chameides L, et
al. Part 12: Pediatric Advanced Life Support: 2015 American
Heart Association Guidelines Update for Cardiopulmonary
Resuscitation and Emergency Cardiovascular Care.
Circulation. 2015;132:S526-42.
3. Panchal AR, Berg KM, Hirsch KG, et al.
2019 American Heart Association Focused Update on Advanced
Cardiovascular Life Support: Use of Advanced Airways,
Vasopressors, and Extracorporeal Cardiopulmonary
Resuscitation During Cardiac Arrest: An Update to the
American Heart Association Guidelines for Cardio-pulmonary
Resuscitation and Emergency Cardiovascular Care.
Circulation. 2019;140:e881-e94.
4. Voelckel WG, Lurie KG, McKnite S, et
al. Effects of epinephrine and vasopressin in a piglet model
of prolonged ventricular fibrillation and cardiopulmonary
resuscitation. Crit Care Med. 2002;30:957-62.
5. Mann K, Berg RA, Nadkarni V.
Beneficial effects of vasopressin in prolonged pediatric
cardiac arrest: a case series. Resuscitation.
2002;52:149-56.
6. Carroll TG, Dimas VV, Raymond TT.
Vasopressin rescue for in-pediatric intensive care unit
cardiopulmonary arrest refractory to initial epinephrine
dosing: A prospective feasibility pilot trial. Pediatr Crit
Care Med. 2012;13: 265-72.
7. Fiser DH, Long N, Roberson PK, Hefley
G, Zolten K, Brodie-Fowler M. Relationship of pediatric
overall performance category and pediatric cerebral
performance category scores at pediatric intensive care unit
discharge with outcome measures collected at hospital
discharge and 1- and 6-month follow-up assessments. Crit
Care Med. 2000; 28:2616-20.
8. Zaritsky A, Nadkarni V, Hazinski MF,
et al. Recommended Guidelines for Uniform Reporting of
Pediatric Advanced Life Support: The Pediatric Utstein
Style. Ann Emerg Med. 1995; 26:487-503.
9. Reis AG, Nadkarni V, Perondi MB, Grisi
S, Berg RA. A prospective investigation into the
epidemiology of in-hospital pediatric cardiopulmonary
resuscitation using the international Utstein reporting
style. Pediatrics. 2002;109: 200-9.
10. Jacobs I, Nadkarni V, Bahr J, et al.
Cardiac Arrest and Cardiopulmonary Resuscitation Outcome
Reports: Update and Simplification of the Utstein Templates
for Resuscitation Registries: A Statement for Healthcare
Professionals from a Task Force of the International Liaison
Committee on Resuscitation (American Heart Association,
European Resuscitation Council, Australian Resuscitation
Council, New Zealand Resuscitation Council, Heart and Stroke
Foundation of Canada, Inter American Heart Foundation,
Resuscitation Councils of Southern Africa). Circulation.
2004;110:3385-97.
11. Naranjo CA, Busto U, Sellers EM, et
al. A method for estimating the probability of adverse drug
reactions. Clin Pharmacol Ther. 1981;30:239-45.
12. Rathore V, Bansal A, Singhi SC,
Singhi P, Muralidharan J. Survival and neurological outcome
following in-hospital paediatric cardiopulmonary
resuscitation in North India. Paediatr Int Child Health.
2016;36:141-7.
13. Donoghue AJ, Abella BS, Merchant R,
et al. Cardiopulmonary resuscitation for in-hospital events
in the emergency department: A comparison of adult and
pediatric outcomes and care processes. Resuscitation.
2015;92: 94-100.
14. Ridgeon EE, Bellomo R, Aberegg SK, et
al. Effect sizes in ongoing randomized controlled critical
care trials. Crit Care. 2017;21:132.
15. Skellett S, Biarent D, Nadkarni V.
What works in paediatric CPR? Intensive Care Med.
2018;44:223-6.
16 Agrawal A, Singh VK, Varma A, Sharma
R. Therapeutic applications of vasopressin in pediatric
patients. Indian Pediatr. 2012;49:297-305.
17. Finn J, Jacobs I, Williams TA, Gates
S, Perkins GD. Adrenaline and vasopressin for cardiac
arrest. Cochrane Database Syst Rev. 2019;1:CD003179.
18. Duncan BW, Ibrahim AE, Hraska V, et
al. Use of rapid-deployment extracorporeal membrane
oxygenation for the resuscitation of pediatric patients with
heart disease after cardiac arrest. J Thorac Cardiovasc
Surg. 1998;116: 305-11.
19. Morris MC, Wernovsky G, Nadkarni VM.
Survival outcomes after extracorporeal cardiopulmonary
resuscitation instituted during active chest compressions
following refractory in-hospital pediatric cardiac arrest.
Pediatr Crit Care Med. 2004;5:440-46.
20. Thourani VH, Kirshbom PM, Kanter KR, et al.
Venoarterial extracorporeal membrane oxygenation (VA-ECMO)
in pediatric cardiac support. Ann Thorac Surg.
2006;82:138-44; discussion 144-5.
|
|
|
 |
|

