|
|
|
Indian Pediatr 2021;58: 611-616 |
 |
Efficacy
and Safety of Thalidomide in Patients With Transfusion-Dependent
Thalassemia
|
|
Jagdish Chandra,
1 Nupur
Parakh,1 Sidharth1,
Neha Singh,1 Sunita Sharma,2
Manish Goel,3
Harish Pemde1
From the 1Division of Pediatric Hematology, Department of Pediatrics;
2Department of Pathology; and 3Department of Community Medicine; Lady Hardinge Medical College and associated Kalawati Saran Children
Hospital, New Delhi.
Correspondence to: Dr Nupur Parakh, B-52, Ashoka Niketan, Opposite
Vigyan Vihar, IP Extension II, Delhi 110 092.
Email: [email protected]
Received: January 15, 2021;
Initial review: February 15, 2021;
Accepted: April 21, 2021.
|
|
Objective: To assess the efficacy and safety of thalidomide in
children with transfusion-dependent thalassemia.
Methods: This prospective, single
center, open-label study enrolled children aged 12-18 years, and who
received thalidomide for a duration of 6 months at a starting dose of
2-3 mg/kg/day. Efficacy was assessed by reduction in transfusion
requirement and rate of fall of hemoglobin. Efficacy was classified as
major, moderate and minimal/no response depending on the reduction in
transfusion requirement. Safety was assessed by adverse effects related
to thalidomide.
Results: 37 children [mean
(SD) age, 14.7 (1.8) years were included. Rate of fall of hemoglobin
reduced from a mean of 1.0 (0.24) g/week pre-thalidomide therapy to 0.58
(0.26) g/week after 6 months of thalidomide (P<0.001). 19
children (51.3%) had major response and 12 (32.4%) had moderate
response. In 13.5% and 32.4% children response was observed within the
first and second month of therapy, respectively. 15 (40.5%) children
remained transfusion - free for a median (IQR) time of 6 (3-10) weeks of
thalidomide therapy. Mean serum ferritin (SD) decreased from 1758.9
(835.1) to 1549.6(1016.9) (P<0.001). Mean HbF (SD) showed an
increase from 2.95(2.6) to 49.2(33.3) (P<0.001). In 32 children,
47 adverse events were observed. Common adverse events were constipation
and neutropenia (mostly mild).
Conclusions: Thalidomide resulted
in major/moderate response in majority of children with
transfusion-dependent thalassemia with satisfactory adverse effect
profile.
Keywords: Hemoglobin F, Iron overload,
Transfusion requirement.
|
|
C
oexistence of hereditary persistence of fetal
hemoglobin (HbF) in patients with transfusion-dependent thalassemia
(TDT) reduces the severity of the disease with several of them becoming
non-transfusion dependent. This clinical benefit of increased HbF
appears to be due to a decrease in the imbalance between
b and non-b
chains, resulting in reduction of ineffective erythropoiesis and
hemolysis [1]. Based on these observations, many drugs including
hydroxyurea, butyrate, 5-azacytidine etc have been studied as inducers
of HbF for patients with thalassaemia and sickle cell disease (SCD)
(2-6).
Thalidomide, a drug known for its immuno-modulating
and anti-angiogenic properties, has recently been demonstrated to induce
globin gene expression and to increase the proliferation of erythroid
cells [7]. Experience with use in non-TDT (NTDT) and TDT is limited
[8-10]. A recent study has shown major response (hemoglobin rise >2 g/dL)
in 50% and 71 % at one month and three month of therapy, respectively in
patients with NTDT [11].
In patients with TDT, a recent study showed mean
hemoglobin increase from 8.9 g/dL to 10.5 (1.18) g/dL after 6 months of
thalidomide treatment [12]. Ramanan and Kelkar from Pune have reported
over 50% reduction in serum ferritin in 59 (50%) patients with
thalassemia [13]. This study was thus undertaken to assess the efficacy
of thalidomide in reducing transfusion requirement and iron overload and
to assess its safety in patients with TDT.
METHODS
This prospective single-center open-label study was
conducted in a tertiary care public hospital of India from October, 2019
to April, 2020. The Study included children with TDT aged 12-18 years
enrolled from the thalassemia day care center, after detailed
counselling regarding the study and explaining the adverse effects of
use of thalidomide. Out of 37 patients, 4 had HbE- b-thalassemia,
but were clinically behaving as TDT. No patient in this study was on
hydroxyurea. Those having HIV, hepatitis C or hepatitis B infection,
known neurological problems, known chronic systemic disease,
hypersplenism, and patients with vitamin B12 or folate deficiency were
excluded. Post- pubertal girls were enrolled immediately after menstrual
period. Ethical clearance was obtained from institutional ethics
committee and approval of Drug Controller General of India (DCGI) was
obtained for use of thalidomide for a new indication. A written consent
was obtained from the parents/ caregivers and assent was obtained from
the participating children.
The sample size was calculated using Epi Info (https://www.openepi.com/SampleSize/SSMean.htm).
A sample size of 32 children was calculated considering the current mean
packed red blood cell (RBC) requirement of 220 mL/kg/year and likely
minimum 10% reduction in annual packed RBC requirement when thalidomide
is provided. The sample size was computed considering the two tailed
test with an alpha error of 0.05 and power of 80%. Considering a drop
out of 15%, a final sample size of 37 children was enrolled in the
study.
Detailed history and examination was done at baseline
and during each follow up visit at 2-4 weeks interval. At follow visits,
enquiries were made specifically for consti-pation, sedation and
neurological symptoms. Baseline investigations included complete blood
counts, absolute reticulocyte count (ARC) (using XN-1000 automated
hematology analyzer, Sysmex Corporation). Prothrom-bin time (PT),
activated partial thromboplastin time (aPTT) and d-dimer levels were
performed on STA compact Stago automated coagulo-meter (Diagnostica
Stago). These investigations were repeated every four weeks. Hemo-globin
F (HbF) levels were estimated at baseline using Bio Rad Variant II (BIO
RAD, US) and was repeated at the end of study at 6 months.
The goal of transfusion therapy was to keep
pre-transfusion hemoglobin level between 9-10.5 g/dL. Thalidomide was
started at dose of 2-3 mg/kg for 24 weeks [12]. Thalidomide was used in
rounded-off value and different strengths were also created using empty
capsules containing 25 mg drug. If the patient was showing response and
was free of adverse effect, the same dose was continued. The dose was
increased upto 3-4 mg/kg in cases with no response with initial dose if
the drug was well tolerated (maximum dose given to patients was 3.7
mg/kg/day). Ecosprin was not given to any patient enrolled for the
study, irrespective of dose of thalidomide, except for the patient who
were splenecto-mized or transiently for patients with increased
D-dimer, during monitoring.
The response to thalidomide therapy was also assessed
as mean change in rate of fall of hemoglobin and transfusion requirement
during study period. The levels of pre-transfusion hemoglobin, fetal
hemoglobin, ARC and serum ferritin were also compared. Subjects with
more than 50% reduction in transfusion requirement as compared to
pre-study transfusion requirement were classified as having major
response (Group 1); those with 25-50% reduction in transfusion
requirement were classified as moderate response (Group 2), and those
with less than 25% decrease in transfusion requirement were classified
as minimal/no response (Group 3).
Statistical analysis: The response was
statistically analyzed, using paired t test. In the three groups,
response was compared using ANOVA test. Post-hoc analysis was also
performed for finding out the statistically significant differences.
P value of less than 0.05 was considered as statistically
significant.
RESULTS
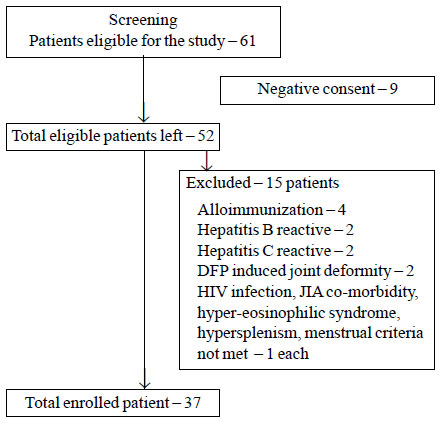
The flow of the study is shown in Fig. 1. The
study included 37 children (M: F-2.36:1) with a mean age of 14.7 (1.82)
years. Table I describes baseline parameters of study subjects.
Notably, only one child was splenectomized, and none had HIV, Hepatitis
B or HCV infection. Mean (SD) HbF level of the subjects was 2.95%
(2.6%). Of the 33 children for whom information on mutation study was
available, seven children had variable combination of
b0/b0
mutations, 9 patients had severe b+/severe
b+
muta-tions, and 5 patients were compound heterozygous for
b0 and severe b+
mutations. Seven patients had variable combi-nation of either severe
b+/
mild b+
or compound hetero-zygous for b0
mutations with second mutation being uncommon
Indian mutation which could not be detected. None of the subjects had
low serum folate or vitamin B12 levels.
 |
|
Fig. 1 Study flow chart.
|
Table I Baseline Characteristics of Children With Thalassemia Enrolled for the Study (N=37)
| Characteristics |
Value |
| Age (y) |
14.7 (1.8) |
| M: F |
26:11 (2.36:1) |
|
Packed red cell received,a mL/kg |
75.7 (12.3) |
|
Pre-transfusion hemoglobin, mg/dL |
9.45 (0.67) |
|
Serum ferritin, ng/mL |
1758.9 (835.1) |
|
Absolute reticulocyte count |
24076.8 (27781.3) |
|
Fetal hemoglobin (%) |
2.95 (2.6) |
| Mutations (n=33)b |
|
| B0/ Severe b+ |
28 |
| E b-double heterozygous |
4 |
| Thalidomide dose (mg/kg/day) |
2.05 (0.35) |
| Chelation, no. |
|
|
Deferasirox
|
14 |
|
Deferasirox and deferiprone |
23 |
|
Serum folate (ng/mL) |
24.4 (14.9) |
|
Serum vitamin B12 (pg/mL) |
421.1 (147.2) |
| Data expressed as mean (SD) or
as stated. aSix-month pre-study. bMutation report of 4 patients
were not available, and 1 patient did not have any of the common
mutations. |
Table II Patient Characteristics at Baseline and on follow-up in Children With Thalassemia Treated With Thalidomide
| Characteristic |
Baseline |
End of the study |
|
Hemoglobin, mg/dL (6 mo) |
9.45 (0.67) |
8.89 (0.6) |
| Transfusion requirement, |
75.7 (12.3) |
38.9 (19.1) |
|
mL/kg (for 6 mo) |
|
|
|
ROF of hemoglobin, (g/wk)
|
1.0 (0.24) |
0.58 (0.3) |
|
Absolute reticulocyte counta |
24076.8 |
111518 |
|
(27781.3) |
(50236.8) |
|
Serum ferritin, ng/mL |
1758.9 |
1549.6 |
|
(835.1) |
(1016.9) |
| Hemoglobin, F (%) |
2.95 (2.6) |
49.2 (33.3) |
|
Data expressed as mean (SD). ROF- Rate of fall. All P<0.001
except aP=0.06. |
Table II describes changes observed in study
parameters as compared to baseline parameters. Rate of fall of
hemoglobin decreased from a mean of 1.0 (0.24) g/week to 0.58 (0.26)
g/week (P<0.001). Hemoglobin F levels showed a significant
increase and serum ferritin decreased significantly (P<0.001).
However, rise in mean ARC was statistically not significant. In 32/37
(86.5%) patients, the dose of drug was increased if they had tolerated
the drug well without any evidence of adverse effects. In 27/37 (72.9%)
patients, dose reduction was done for development of adverse effects on
any follow up visit, but most of the adverse effects were either grade 1
or 2 [14].
Of the 37 patients recruited in the study, one child
succumbed to dengue shock syndrome during second month of study period.
In two children, therapy was discontinued due to withdrawal of consent
and adverse effect in one case each. Nineteen children (51.3%) had major
response while 12 children (32.4%) had moderate response; remaining 6
(16.2%) had minimal/no response. Table III shows that before
intervention, the three groups were similar with respect to their mean
pre-transfusion hemoglobin and mean packed RBC received in 6 months
preceding the study period. However, during the study period, the group
with best response received 25.02 (10.37) mL/kg packed RBC compared to
the group with minimal/no response receiving 67.76 (16.31) mL/kg (P<0.001).
Mean HbF in the group with best response was 66.9% (28.59%) while in
group 3 it was only 16.62% (11.23%) (P<0.001). Although mean
serum ferritin was significantly decreased, the fall in individual
groups was not statistically significant.
Table III Study Parameters in Children With Thalassemia Based on Response to Thalidomide
| Parameter |
Major response (n=19) |
Moderate response (n=12) |
Mild response (n=6) |
|
Pre-transfusion hemoglobin, mg/dL
|
|
|
|
| Pre-study |
9.53 (0.61) |
9.47 (0.67) |
9.12 (0.87) |
|
At the end of studya |
9.2 (0.58) |
8.65 (0.51) |
8.39 (0.36) |
|
pRBC received, mL/kg |
|
|
|
| 6 mo pre-study |
73.96 (12.9) |
77.8 (10.9) |
77.1 (14.1) |
|
6 mo during studyc |
25.0 (10.4) |
48.8 (8.9) |
67.8 (16.3) |
| Thalidomide, mg/kg/d |
2.53 (0.36) |
2.28 (0.49) |
2.56 (0.35) |
|
ROF of Hb (g/wk)a |
0.45 (0.17) |
0.67 (0.29) |
0.8 (0.19) |
| Hemoglobin F (%) |
|
|
|
| Pre-therapy |
3.5 (2.2) |
2.25 (3.0) |
2.68 (3.0) |
|
Post therapyb |
66.9 (28.6) |
39.74 (32.0) |
16.62 (11.2) |
|
Serum ferritin, ng/mL |
|
|
|
| Initial |
1648.8 (700.4) |
1867.4 (1237.5) |
1890.4 (640.6 ) |
| At the end of study |
1314.4 (718.6) |
1694.5 (1177.2) |
1886.7 (1365.0) |
| Data expressed as mean (SD).
Hb- Hemoglobin, pRBC-Pure red blood cell, ROF- Rate of fall. aP<0.01,
bP=0.001; cP<0.001. |
In five children (13.5%) response was observed within
first month of therapy, 12 more responded in the second month. Response
was observed in third and fourth month of therapy in additional 5
(13.51%) and 6 (16.21%) patients, respectively. During the study period,
15 (40.5%) children remained free of transfusion for a median (IQR) time
of 6 (3-10) weeks of thalidomide therapy. However, after stopping
thalidomide therapy, all children have required transfusions after a
median (IQR) of 24 (19-52) days.
A total of rest 32 children had 47 adverse events;
constipation being the most common (14, 37.8%). Raised transaminases in
two children were considered unrelated, as they were also receiving
deferasirox. Other adverse effects included somnolence/sedation (n=3)
and mild dizziness (n=5, in one child this necessitated
disconti-nuation of therapy). One child developed acute kidney injury
during study period. This child was also receiving deferasirox, but as
renal injury occurred during the study period, thalidomide was
discontinued. Neutropenia was observed in 10 children; however, only one
child had absolute neutrophil count less than 500/mm3,
which required temporary cessation of thalidomide. D-dimer was elevated
in 6 (16.2%) children but none had any features suggestive of
thromboembolism. Infections occurred during study period in 8 subjects:
pneumonia, 2; chicken-pox, 1; unclassified acute febrile illness, 3; and
dengue infection in 2 (one of whom died of dengue shock syndrome). The
patient who died of dengue shock syndrome, the starting dose of
thalidomide was 1.6 mg/kg, upto maximum of 2.4 mg/kg in follow-up visit.
That child was not splenectomized, never had neutropenia during the
study period, and was on deferasirox alone. During the febrile period,
thalidomide had been withheld.
All adverse events were grade1 to grade 2 except one
episode of neutropenia (grade 3) and one episode of acute kidney injury
(grade 4), necessitating temporary cessation of the drug. No female
patient in our study population had any menstrual abnormality. One child
withdrew from the study due to sedation interfering with his studies.
This child was on starting dose of 2.4 mg/kg thalidomide which was
reduced after grade 2 sedation.
Nerve conduction studies (NCV) were not performed
routinely at baseline or after therapy. Only one child complained of
mild tingling sensation, for which NCV was performed, and thalidomide
was restarted as it was normal. His serum B12 and folate levels were
normal.
DISCUSSION
Over the last decade, there has been an interest in
use of thalidomide in patients with thalassemia syndromes. After initial
isolated case reports, it was used with success in patients with NTDT.
In TDT, the experience is limited and is now emerging. Jiskani and Memon
[12] reported good response in 70 children but the extent of decrease in
transfusion requirement was not commented upon. Yassin [15] described
his results on 37 patients including adults and only 14 patients with
TDT. He described response in over 75% cases. He also describes the fall
in transfusion requirement in terms of ‘units’ of packed cells and not
in mL/kg [15]. Other studies from India and China have also reported
response in up to 70% patients [16-18].
The present study is exclusively on children with
TDT. We included children above 12 years as FDA approval is restricted
to 12 years or above [19]. We have demonstrated major and moderate
response in 51% and 32 % patients with reduction in transfusion
requirement coming up as early as first month of therapy. The response
rates and timing of response are similar to earlier studies [15,16,20].
We assessed the weekly rate of fall of hemoglobin which decreased
significantly, as well as a decrease in packed cell requirement. Of the
responders, 15 patients remained transfusion free after a median (IQR)
of 6 (3-10) weeks. However, all our patients have started requiring
transfusions after stopping thalidomide.
The response to thalidomide is described to be by
production of fetal Hb. There are experimental studies demonstrating
increased HbF production with thalido-mide and other related compounds
[7,21,22]. Clinical studies have not looked at rise in HbF; though, we
found a rise in HbF in those with major response. Thalidomide also seems
to have effect on iron overload. We observed a modest but significant
fall in mean serum ferritin; alth-ough, all the patients continued to
receive chelation. This is in concordance with earlier observations
[12,13,15].
Therapy with thalidomide was well-tolerated. Nag, et
al. [16] observed constipation in over 40% cases. Shah, et al. [17]
described thrombocytopenia in 66%, but their patients were also
receiving hydroxyurea. Neutropenia was reported in 5% patients in
another study [20]. However, we encountered neutropenia in 10 (27%)
cases one of which was severe necessitating temporary cessation. Out of
10 study patients who developed low ANC during the study period, 6/23
patients were on DFX and DFP and 4/14 patients were on DFX. However,
risk of development of neutropenia between the two group (on combined
DFP and DFX and on DFX alone was not statistically significant (P=0.87).
In a study by Naithani, et al. [23] on safety of deferiprone in
children, neutropenia was observed only in 2/44 patients. However, as
DFP can also cause neutropenia, children on deferiprone and thalidomide,
they need a closer watch on their blood counts. One concern that we have
is occurrence of infections in 8 subjects over the study period. It is
unclear whether this is a chance occurrence or related to thalidomide
administration. This is not described as a known adverse effect of
thalidomide.
The study has certain limitations. We have not
studied different doses. Moreover, follow up after stoppage of drug was
not a part of the study.
Therapy with thalidomide is being looked at as an
affordable alternative to transfusion therapy or at least to partially
offset the transfusion needs [24]. Being relatively inexpensive and well
tolerated also makes it a viable option. However, for a drug to be
administered indefinitely there are certain questions which need to be
addressed. First, studies are required to find out most effective and
safe dose. Second, whether the drug should be continued in full doses or
doses can be reduced after a response is obtained. Third, criteria for
response need to be defined and applied uniformly. Role of intermittent
therapy also needs to be explored. Safety under 12 years is also not
been studied. Interactions with DFX and DFP- two commonly administered
iron chelators also need to be studied. Larger studies to answer these
issues are required before decision for long term routine use is taken.
Till such time drug should be used under strict monitoring of the
patients.
Ethics clearance: Institutional ethics committee,
Lady Hardinge Medical College; No. LHMC/ECHR/2019/29 dated September 23,
2019. DCGI Clearance: F. No. 12-01/19-DC (Pt-208) dated November 20,
2019.
Contributors: JC: conceived and designed the
study, drafted the manuscript; NP: reviewed the literature, collected
the data, helped in drafting the manuscript; S and NS collected the
data, SS supervised the laboratory work; MG: did statistical analysis:
HP: helped in study design. All authors approved the final version of
manuscript, and are accountable for all aspects related to the study.
Funding: National Thalassemia Welfare Society of
India supplied thalidomide throughout the study period. The Society also
funded the insurance of patients during the study period. Competing
interest: None stated.
|
What is already Known
•
Thalidomide induces globin gene expression and increases the
proliferation of erythroid cells.
WHAT THIS STUDY ADDS?
•
Thalidomide can be an effective drug to reduce transfusion
requirement in children with transfusion-dependent thalassemia.
|
REFERENCES
1. Wood WG, Weatherall DJ, Clegg JB. Interaction of
heterocellular hereditary persistence of fetal haemoglobin with beta
thalassaemia and sickle cell anaemia. Nature. 1976;264:247-49.
2. Charache S, Terrin ML, Moore RD, et al. Effect of
hydroxyurea on frequency of painful crises in sickle cell anaemia. N
Engl J Med. 1995;332:1317-322.
3. Hajjar FM, Pearson HA. Pharmacologic treatment of
thalassemia intermedia with hydroxyurea. J Pediatr. 1994; 125:490.
4. Candido EP, Reeves R, Davie JR. Sodium butyrate
inhibits histone deacetylation in cultured cells. Cell. 1978;14: 105-13.
5. Ley TG, De Simone J, Anagnou NP. 5-azacytidine
selectively increases gamma-globin synthesis in a patient with beta+
thalassemia. N Engl J Med. 1982;307:1469.
6. De Simone J, Koshy M, Dorn L, et al. Maintenance
of elevated fetal hemoglobin levels by decitabine during dose interval
treatment of sickle cell anemia. Blood. 2002;99: 3905-908.
7. Aerbajinai W, Zhu J, Gao Z, et al. Thalidomide
induces ã-globin gene expression through increased reactive oxygen
species-mediated p38 MAPK signaling and histone H4 acetylation in adult
erythropoiesis. Blood. 2007;110: 2864-871.
8. Masera N, Tavecchia L, Capra M, et al. Optimal
response to thalidomide in a patient with thalassemia major resistant to
conventional therapy. Blood Trans. 2010;8:63-65.
9. Li Y, Ren Q, Zhou Y, et al. Thalidomide has a
significant effect in patients with thalassemia intermedia. Haemato-logy.
2018;23:50-54.
10. Ricchi P, Costantini S, Spaciano A, et al. The
long term and extensive efficacy of low dose thalidomide in a case of
untransfusable case of non-transfusion dependent thalassemia. Blood
Cells, Molecules and Dis. 2016;57:97-99.
11. Ren Q, Zhou YL, Wang L, et al. Clinical trial on
effect of thalidomide on hemoglobin synthesis in patients with moderate
thalassemia intermedia. Ann Haematology 2018; 97:1933-939.
12. Jiskani SA, Memon S. Effect of thalidomide in
patients with b-thalassemia
major. Hematology Trans International J. 2018;6:34-36.
13. Ramanan V, Kelker K. Role of thalidomide in
treatment of beta thalassemia. J Blood Dis Med. 2017.
14. Ghobrial IM, Rajkumar SV. Management of
thalidomide toxicity. J Support Oncol. 2003;1:194-205.
15. Yassin AK. Promising response to thalidomide in
symptomatic b-thalassemia.
Indian J Hematol Blood Transfus. 2020;36:337-41.
16. Nag A, Radhakrishnan VS, Kumar J, et al.
Thalidomide in patients with transfusion dependent E-b
thalassemia refractory to hydroxyurea: A single center study. Indian J
Hematol Blood Transfus. 2020;36:399-402.
17. Shah S, Sheth R, Shah K, et al. Safety and
effectiveness of thalidomide and hydroxyurea combination in
b thalassemia
intermedia and major: A retrospective pilot study. British J Haematol.
2020;188:e18-e21.
18. Yang K, Wu Y, Zhou Y, et al. Thalidomide for
patients with b-thalassemia:
A multicenter experience. Mediterranean J Hematol Infect Dis.
2020;12:e2020021.
19. Label (PDF) FDA. Accessed November 26, 2020.
Available from: www.accessdata.fda
20. Mehta P, Yadav N, Soni P, et al. Experience with
low dose thalidomide in transfusion dependent beta thalassemia in
resource limited setting. Blood. 2019;134:963.
21. Parseval LAM, Verhelle D, Glezer E, et al.
Pomalidomide and linelidomide regulate erythropoiesis and fetal
hemoglobin production in human CD34+ cells. J Clin Invest.
2008;118;248-58.
22. Jalaji FMA, Fard AD, Hajizamani S, et al.
Thalidomide is more efficient than sodium butyrate in enhancing GATA-1
and EKLF gene expression in erythroid progenitors derived from HSCs with
B globin gene mutation. Int J Hematol Oncol Stem Cell Res. 2016;
10:37-41.
23. Naithani R, Chandra J, Sharma S. Safety of
oral iron chelator deferiprone in young thalassaemics. Eur J Haematol.
2005;74:217-20.
24. Naithani R, Jeyaraman P, Mohapatra M. Alternative
strategies in thalassemia: Focus on thalidomide. Indian J Hematol Blood
Transf. 2020;36:27-28.
|
|
|
 |
|

