|
|
|
Indian Pediatr 2015;52:
573-578 |
 |
Enteral paracetamol or Intravenous
Indomethacin for Closure of Patent Ductus Arteriosus in Preterm
Neonates: A Randomized Controlled Trial
|
|
Swarup Kumar Dash, Nandkishor S Kabra, Bhupendra S
Avasthi, Shobha R Sharma,
Phalguni Padhi and Javed Ahmed
From Department of Neonatology, Surya Children’s
Hospital, Mangal Ashirwad, Santacruz West, Mumbai, India.
Correspondence to: Dr Nandkishor S Kabra, Department
of Neonatology, Surya Children’s Hospital, Mangal Ashirwad, Junction of
S V Road and Dattatraya Road, Santacruz West, Mumbai 400 054, India.
Email: [email protected]
Received: August 06, 2014;
Initial review: October 04, 2014;
Accepted: April 21, 2015.
|
Objective: To compare the efficacy of enteral paracetamol and
intravenous indomethacin for closure of patent ductus arteriosus (PDA)
in preterm neonates.
Design: Randomized controlled
trial.
Setting: Level III neonatal
intensive care unit.
Participants: 77 preterm neonates
with birth weight £1500
g and PDA size ³1.5
mm, with left to right ductal flow with left atrium to aortic root ratio
>1.5:1; diagnosed by 2D-Echo within first 48 hours of life.
Intervention: Paracetamol drops
through the infant feeding tube (15mg/kg/dose 6 hourly for 7 days) or
intravenous indomethacin (0.2 mg/kg/dose once daily for 3 days).
Outcome measures: Primary:
PDA closure rate assessed by echocardiography. Secondary: need
for surgical closure of PDA, renal impairment, gastrointestinal bleed,
necrotising enterocolitis, hepatotoxicity, pulmonary hemorrhage, sepsis,
hypothermia, retinopathy of prematurity, intraventricular hemorrhage,
bronchopulmonary dysplasia and mortality.
Results: PDA closure rate was
100% (36/36) in enteral paracetamol group as compared to 94.6% (35/37)
in intravenous indomethacin group (P=0.13). The secondary
outcomes were also similar between the two groups. There was no
occurrence of hepatotoxicity.
Conclusions: Enteral paracetamol
is safe but not superior to intravenous indomethacin in the treatment of
PDA in preterm neonates.
Key words: Echocadiography,
Neonate, Patent ductus arteriorus, Treatment.
Trial Registration: CTRI/2012/12/003/63.
|
|
D
uctus arteriosus may close spontaneously by day 7
of life in only 70% of infants with birth weight between 1000 to 1500 g
and 30%-35% of infants with birth weight <1000 g [1,2]. If the patent
ductus arteriosus (PDA) is hemodynamically significant and symptomatic,
therapeutic interventions may be required to facilitate its closure
[3,4]. Reported efficacy rate for ductal closure using both indomethacin
and ibuprofen is about 60% to 80 % [5]. However, both indomethacin and
ibuprofen have been associated with potential adverse effects including
peripheral vasoconstriction, gastrointestinal perforations, necrotizing
enterocolitis (NEC), renal impairment, platelet aggregation dysfunction,
and hyperbilirubinemia [6-11].
Paracetamol is efficacious in closure of PDA in
preterm infants [12-14]. It acts mainly by inhibiting the peroxidase
enzyme activity. Peroxidase is activated at lower peroxide concentration
than that of cyclo-oxygenase, suggesting that paracetamol may work well
at decreased peroxide concentrations like in hypoxia [12-14]. It also
has a wide margin of safety, but there is paucity of controlled trials
comparing paracetamol with indomethacin for closure of PDA.
This study compared the efficacy and safety of
enteral paracetamol with intravenous indomethacin in closure of
hemodynamically significant PDA in preterm neonates.
Methods
This open-label randomized controlled trial was
conducted at a level III neonatal intensive care unit (NICU) of a
private hospital in Mumbai, India. The study was approved by hospital’s
local academic research and ethics committee. Written informed consent
was obtained from the parents prior to enrolment of their infants.
Inclusion criteria were: (i) preterm infant with birth weight
£1500 grams
and (ii) echocardiography performed within the first 48 hours of
life demonstrating PDA size ³1.5
mm at the narrowest diameter, left to right shunt across the duct and
ratio of the diameter of the left atrium to that of the aortic root
(LA:AO) >1.5:1. Exclusion criteria were: (i) inability to
administer the study drug within 48 hours of birth, (ii)
structural duct-dependent congenital heart disease, renal disease (such
as multicystic dysplastic kidney and polycystic disease of kidney), (iii)
dysmorphic features or congenital anomalies likely to affect
life-expectancy or neurologic development, (iv) maternal
tocolytic therapy with indomethacin or another prostaglandin inhibitor
within 72 hrs prior to delivery, (v) overt clinical bleeding at
more than one site, (vi) Platelet count <50×109/L,
(vii) hydrops fetalis, and (viii) infant not considered
viable.
An echocardiogram which included doppler flow studies
was performed by a trained pediatric cardiologist within 48 hours of
birth to look for presence of any hemodynamically significant PDA. PDA
was considered hemodynamically significant if size was
³1.5 mm at the
narrowest diameter [15,16], left to right shunt was seen across the duct
and the LA:AO ratio was more than 1.5:1. The study period was from March
2012 to September 2013. All the relevant data was collected using a
predesigned case record form.
All eligible neonates meeting the inclusion criteria
were randomized into two groups, using a 1:1 ratio. Random sequence
generation was performed by using random allocation software in variable
blocks of 2 or 4. This sequence was generated by a statistician who was
not part of the study. Allocation concealment was done by sequentially
numbered sealed opaque envelopes. When a patient meeting the inclusion
criteria was ready to be enrolled in the study, the doctor on duty
obtained written informed consent from the parents. The serially
numbered opaque sealed envelope was opened by the doctor and the patient
was enrolled into the respective intervention group.
As per randomization, patients received paracetamol
drops (Calpol drops, 100 mg/mL, Glaxo SmithKline) through the infant
feeding tube at a dose of 15 mg/kg/dose four times daily for 7 days (28
doses) or IV indomethacin (1mg/mL, Lygacin IV, Alliance Overseas) at a
dose of 0.2 mg/kg/dose, diluted with normal saline to make 5 mL solution
and infused over 20 minutes by syringe pump once daily for three days
[17]. As per study protocol, two additional extra doses of indomethacin
were allowed in the indomethacin group, if clinical evaluation after
three doses showed persistence of PDA as demonstrated by clinical signs
and symptoms such as tachycardia, wide pulse pressure and persistent
murmur.
The primary outcome measure of the study was PDA
closure. The first screening echocardiography was performed
within 48 hours of life. Subsequent follow-up echocardiography was
performed after completion of 7 days from initiation of treatment. The
PDA was considered to be closed if there was no evidence of any flow in
the ductus arteriosus on echocardiographic and doppler flow assessment.
Serum electrolyte and serum creatinine values were measured before
starting treatment with the drug, and subsequently thereafter at regular
intervals as per standard unit policy. Urine output was measured daily.
Renal impairment was defined as presence of either oliguria (urine
output of < 0.5 mL/kg/hr) over a 6 hour period or serum creatinine
levels more than twice the age appropriate norms. Gastro-intestinal (GI)
bleeding was defined as the presence of blood- stained or coffee ground
brown gastric aspirates. Mild gastric aspirate was defined as
blood-stained or altered brownish blood in the aspirate, and major GI
bleeding was defined as presence of frank blood in the gastric aspirate.
Necrotising enterocolitis (NEC) was diagnosed as per modified Bell’s
staging [18]. Liver function tests were measured on day 7 of life;
hepatotoxicity was defined, if the hepatic enzymes were elevated more
than twice of the normal reference values. Pulmonary hemorrhage was
diagnosed if a blood tinged tracheal aspirate was obtained. Positive
early- and late-onset sepsis screen was defined as positive C-reactive
protein (CRP) before and after first 72 hours of life (CRP >6 mg/L),
respectively. Early-onset sepsis was defined as isolation of pathogenic
organism from a blood culture collected in first 72 hours of life. Late
onset sepsis was defined as isolation of pathogenic organism from a
blood culture collected after first 72 hours of life. All blood cultures
were collected in BacT/ALERT 3D (Bio-merieux) blood culture bottles.
Hypothermia was defined as occurrence of temperature
£36º celsius during
the therapy period. Retinopathy of prematurity (ROP) was classified as
per the International classification of retinopathy [19]. ROP needing
either laser or anti-VEGF (Avastin) therapy was labelled as severe ROP.
Neuro-sonography was performed as per our unit protocol, at least twice;
first sonography between day 5 to 7 of life and second sonography
between days 21 to 28 of life. A third cranial ultrasonography was
performed if an infant was still admitted to the NICU at 36 weeks
corrected gestational age. Grading of intraventricular hemorrhage (IVH)
was performed according to the Papile grading system [20], and features
of periventricular leukomalacia (PVL) were also assessed. Requirement of
supplemental oxygen at 28 days of postnatal age was assessed.
Bronchopulmonary dysplasia (BPD) /chronic lung disease (CLD) was defined
by the need for supplemental oxygen at 36 weeks of postmenstrual age
[21]. The success rate of indomethacin for closure of PDA was estimated
to be 50 percent [22], and a sample size of 72 (36 in each group) was
calculated to be adequate for a 30% difference with a two-sided alpha
error of 0.05 and beta error of 0.2 (power 80%).
To compare the outcome variables on continuous and
ordinal scale, two sample t tests or the Mann Whitney test were used. To
compare the outcome variables on nominal type of data, Fisher exact test
was used. Relative risk (RR) and 95% CI were calculated as a measure of
association for the dichotomous outcomes. The analysis was performed by
applying the intention to treat principle. Analysis was performed by
using IBM SPSS 21 software.
Results
A total of 171 premature neonates with birth weight
<1500 g were
admitted in NICU during the study period and were assessed within 48
hours of birth for presence of PDA. Out of these, 38 were randomized to
the enteral paracetamol group and 39 were randomized to the intravenous
indomethacin group (Fig. 1). Two infants in each group
died before the time of assessment of PDA closure by echocardiography.
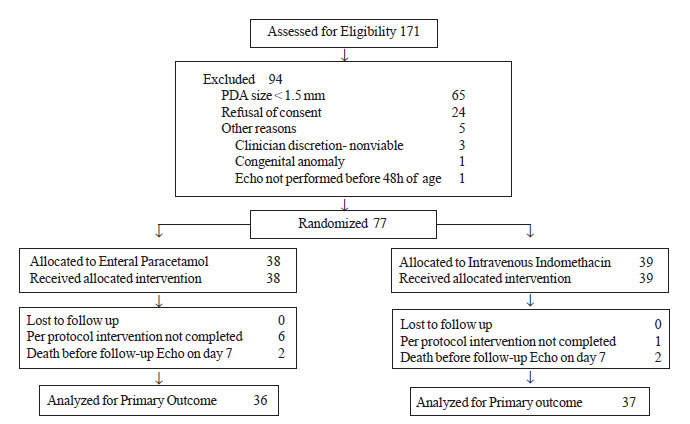 |
|
Fig.1 Participant flow in the study.
|
In the enteral paracetamol group, six neonates (GI
bleeding 3, NEC 1, deaths 2), and in the intravenous indomethacin group,
one neonate (metabolic acidosis and deteriorating clinical condition)
failed to complete the full intended course of the study drug. One
patient in the indomethacin group received 2 extra doses as clinical
examination performed after 3 doses revealed persistence of PDA. There
were no significant differences in the baseline characteristics of the
mothers and their infants between the two study groups (Table
I).
TABLE I Baseline Characteristics of the Study Participants
|
Characteristics |
Paracetamol |
Indomethacin |
|
Group (n=38) |
Group (n= 39) |
|
Mother |
|
|
|
*Age, y |
31.6 (5.3) |
31.2 (4.9) |
|
Preeclampsia/Eclampsia |
10 (26.3) |
12 (30.7) |
|
Tocolysis <7d before delivery |
2 (5.2) |
3 (7.6) |
|
Antenatal glucocorticoids |
33 (86.8) |
31 (79.4) |
|
Caesarean delivery |
23 (60.5) |
28 (71.7) |
|
Infant |
|
|
|
*Gestational age, wk |
28.5 (2.7) |
28.9 (2.6) |
|
Gestational age £27 weeks |
14 (36.8) |
11 (28.2) |
|
*Birth weight, g |
989 (299) |
1027 (262) |
|
AGA |
26 (68.5) |
33 (84.6) |
|
SGA |
12 (31.5) |
6 (15.4) |
|
Male gender |
14 (36.8) |
13 (33.3) |
|
Singleton |
22 (57.9) |
20 (51.3) |
|
#APGAR, 1 min |
6 (5-6) |
6 (5-7) |
|
#APGAR, 5 min |
7.5 (7-8) |
8 (7-8) |
|
Surfactant |
33 (86.8) |
33 (84.6) |
|
*1st Echo age in h |
14.7 (8.4) |
15.9 (11.8) |
|
*PDA size in mm |
2.02 (0.42) |
2.11 (0.53) |
|
Mechanical ventilation |
19 (50) |
21 (54) |
|
CPAP |
12 (32) |
14 (36) |
|
Oxygen by hood |
7 (18) |
4 (10) |
|
Values in *mean (SD); #Median (IQR); Rest all in No.(%). |
Table II compares the outcomes in two study
groups. There was no significant difference in the PDA closure rate
between the two groups. None of the infants in either group required
surgical closure of PDA.
TABLE II Comparison of PDA Closure Rate and Adverse Events With Paracetamol and Indomethacin
|
Outcomes |
Paracetamol Group |
Indomethacin Group |
RR (95% CI) |
P |
|
No. /Total No. (%) |
No. /Total No. (%) |
|
|
|
PDA Closure |
36/36 (100) |
35/37 (94.6) |
1.05 (0.96-1.16) |
0.49 |
|
Secondary Outcomes |
|
|
|
|
|
Renal impairment |
1/38 (2.6) |
0/39 (0) |
|
0.49 |
|
GI Bleed |
10/38 (26.3) |
7/39 (17.9) |
1.47 (0.62-3.45) |
0.38 |
|
NEC (all grades) |
2/38 (5.3) |
4/39 (10.3) |
0.51 (0.10-2.64) |
0.42 |
|
Early onset sepsis - screen positive |
21/38 (55.3) |
17/39 (43.6) |
1.26 (0.80-2.00) |
0.31 |
|
Early onset sepsis -blood culture positive |
1/38 (2.6) |
0/39 (0) |
|
0.49 |
|
Pulmonary hemorrhage |
3/38 (7.9) |
0/39 (0) |
|
0.99 |
|
ROP (all grades) |
24/29 (82.8) |
26/30 (86.7) |
0.95 (0.77-1.19) |
0.68 |
|
Severe ROP needing treatment |
8/29 (27.6) |
7/30 (23.3) |
1.18 (0.49-2.84) |
0.71 |
|
IVH all grades and PVL |
8/37 (21.6) |
7/38 (15.6) |
1.17 (0.47-2.09) |
0.73 |
|
O2 requirement at 28 d |
13/27 (48.1) |
17/31 (54.8) |
0.88 (0.53-1.48) |
0.61 |
|
O2 requirement at ≥36
wk |
5/27 (18.5) |
6/30 (20.0) |
0.93 (0.32-2.69) |
0.89 |
|
Death |
8/38 (21.1) |
8/39 (20.5) |
1.02 (0.43-2.45) |
0.95 |
|
PDA: Patent ductus arteriosus; NEC: necrotizing
enterocollitis; ROP: retinopathy of prematurity; IVH:
intraventricular hemorrhage; PVL: periventricular leucomalacia;
GI: gastrointestinal. |
Discussion
The results of our study suggest that enteral
paracetamol is safe but not superior to intravenous indomethacin in
promoting closure of the hemodynamically significant PDA in premature
infants when treatment commences in the first 48 hours after diagnosis
by echocardiography and Doppler. Our study did not find any significant
difference in the frequency of adverse events, outcomes including GI
bleed, NEC, ROP, IVH/PVL, pulmonary hemorrhage and CLD/BPD.
The main limitation of our study was lack of blinding
of the caregivers to the study intervention. Also, it is possible that
some of our neonates might have had spontaneous PDA closure during the
first 7 days, as the follow up echocardiographic study was performed
only after completion of full 7 days after initiation of treatment.
Additional limitation of our study is that we have only evaluated
short-term outcomes, in a selected group of premature infants,
one-fourth of whom were SGA. This would significantly affect
generalizability of this study. When we planned the study, we assumed
PDA closure rate of 50% in indomethacin group and 80% in paracetamol
group. On completion of our study we found that PDA closure rate was 95%
in indomethacin group and 100% in paracetamol group. Our study,
therefore, was underpowered to demonstrate this minor difference between
two intervention drugs.
Case series describing use of paracetamol for PDA
have been published [12-14,17,23]. More recently, two randomized
controlled trials comparing oral paracetamol with ibuprofen have been
published [24,25]. Both of these trials documented that paracetamol in
dose of 15 mg/kg/dose every 6 hourly for 3 days had comparable efficacy
(73-81%) to ibuprofen (78-79%), in obtaining PDA closure. In our study,
paracetamol was used for 7 days, and closure rate was almost 100%.
We conclude that oral paracetamol is safe but not
superior to intravenous indomethacin in closure of PDA. In developing
countries, where intravenous indomethacin use is constrained by
scarcity, high cost and difficulty in monitoring the side effects, oral
paracetamol may be considered as an alternative. We recommend studies
with an appropriate sample size, simultaneously looking at long-term
neurodevelopmental outcome effects of paracetamol in treatment of PDA.
Contributors: SKD: review of literature, data
collection and wrote the first draft; NSK: designing of study, drafting
the article, analysis and interpretation of data. He will and will act
as guarantor; BSA, SRS, PP, JA: designing of study, collection of data
and drafting the manuscript. The final manuscript was approved by all
the authors.
Funding : None ; Competing interests :
None stated.
|
What is Already Known?
• Oral paracetamol is comparable to ibuprofen
in terms of PDA closure rate.
What This Study Adds?
• Enteral paracetamol for preterm infants with
hemodynamically significant PDA is safe but not superior to
intravenous indomethacin.
|
References
1. Nemerofsky SL, Parravicini E, Bateman D, Kleinman
C, Polin RA, Lorenz JM. The Ductus arteriosus rarely requires treatment
in infants >1000 grams. Am J Perinatol. 2008; 25:661-6.
2. Koch J, Hensley G, Roy L, Brown S, Ramaciotti C,
Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus
in neonates at a birth weight of 1000 grams or less. Pediatrics.
2006;117:1113-21.
3. Herrman K, Bose C, Lewis K, Laughon M. Spontaneous
closure of the patent ductus arteriosus in very low birth weight infants
following discharge from the neonatal unit. Arch Dis Child Fetal
Neonatal Ed. 2009; 94:F48-50.
4. Jhaveri N, Moon-Grady A, Clyman RI. Early surgical
ligation versus a conservative approach for management of patent ductus
arteriosus that fails to close after indomethacin treatment. J Pediatr.
2010;157:381-7.
5. Van Overmeire B, Smets K, Lecoutere D, Van de
Broek H, Weyler J, Degroote K, et al. A comparison of ibuprofen
and indomethacin for closure of patent ductus arteriosus. N Engl J Med.
2000;343:674-81.
6. Sankaran K, Puckett B, Lee DS, Seshia M, Boulton
J, Qiu Z, et al. Variations in incidence of necrotizing
enterocolitis in Canadian neonatal intensive care units. J Pediatr
Gastroenterol Nutr. 2004;39:366-72.
7. Gersony WM, Peckham GJ, Ellison RC, Miettinen OS,
Nadas AS. Effects of indomethacin in premature infants with patent
ductus arteriosus: Results of a national collaborative study. J Pediatr.
1983;102:895-906.
8. Lago P, Bettiol T, Salvadori S, Pitassi I,
Vianello A, Chiandetti L, et al. Safety and efficacy of ibuprofen
versus indomethacin in preterm infants treated for patent ductus
arteriosus: a randomised controlled trial. Eur J Pediatr.
2002;161:202-7.
9. Vieux R, Desandes R, Boubred F, Semama D,
Guillemin F, Buchweiller MC, et al. Ibuprofen in very preterm
infants impairs renal function for the first month of life. Pediatr
Nephrol. 2010;25:267-74.
10. Ahlfors CE. Effect of ibuprofen on bilirubin-albumin
binding. J Pediatr. 2004;144:386-8.
11. Rheinlaender C, Helfenstein D, Walch E, Berns M,
Obladen M, Koehne P. Total serum bilirubin levels during cyclooxygenase
inhibitor treatment for patent ductus arteriosus in preterm infants.
Acta Paediatr. 2009;98:36-42.
12. Hammerman C, Bin-Nun A, Markovitch E, Schimmel
MS, Kaplan M, Fink D. Ductal closure with paracetamol: A surprising new
approach to patent ductus arteriosus treatment. Pediatrics.
2011;128:e1618-21.
13. Yurttutan S, Oncel MY, Arayicý S, Uras N, Altug
N, Erdeve O, et al. A different first-choice drug in the medical
management of patent ductus arteriosus: Oral paracetamol. J Matern Fetal
Neonatal Med. 2013;26:825-7.
14. Oncel MY, Yurttutan S, Uras N, Altug N, Ozdemir
R, Ekmen S, et al. An alternative drug (paracetamol) in the
management of patent ductus arteriosus in ibuprofen-resistant or
contraindicated preterm infants. Arch Dis Child Fetal Neonatal Ed.
2013;98:F94.
15. Evans N. Diagnosis of patent ductus arteriosus in
the preterm newborn. Arch Dis Child. 1993;68:58-61.
16. Kluckow M, Evans N. Early echocardiographic
prediction of symptomatic patent ductus arteriosus in preterm infants
undergoing mechanical ventilation. J Pediatr. 1995; 127:774-9.
17. Young TE, Mangum B. Neofax ® 2010, 23rd edition,
Montavale NJ, Thomson Reuters; 2010.p.180-1.
18. Bell MJ, Ternberg JL, Feigin RD, Keating JP,
Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis.
Therapeutic decisions based upon clinical staging. Ann Surg.
1978;187:1-7.
19. International Committee for the Classification of
Retinopathy of Prematurity. The International classification of
retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991-9.
20. Papile LA, Burstein J, Burstein R, Koffler H.
Incidence and evolution of subependymal and intraventricular hemorrhage:
A study of infants with birth weights less than 1,500 gm. J Pediatr.
1978;92:529-34.
21. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins
EM. Abnormal pulmonary outcomes in premature infants: Prediction from
oxygen requirement in the neonatal period. Pediatrics. 1988;82:527-32.
22. Knight DB. The treatment of patent ductus
arteriosus in preterm infants. A review and overview of randomized
trials. Semin Neonatol. 2001;6:63-73.
23. Jasani B, Kabra N, Nanavati RN. Oral paracetamol
in treatment of closure of patent ductus arteriosus in preterm neonates.
J Postgrad Med. 2013;59:312-4.
24. Dang D, Wang D, Zhang C, Zhou W, Zhou Q, Wu H.
Comparison of oral paracetamol versus ibuprofen in premature infants
with patent ductus arteriosus: A randomized controlled trial. PLoS One.
2013; 8:e77888.
25. Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N,
Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the
management of patent ductus arteriosus in preterm infants: A randomized
controlled trial. J Pediatr. 2014;164:510-4.
|
|
|
 |
|

