|
|
|
Indian Pediatr 2014;51:
550-554 |
 |
Comparative Short term Efficacy and
Tolerability of Methylphenidate and Atomoxetine in Attention
Deficit Hyperactivity Disorder
|
|
Jasmin Garg, Priti Arun and BS Chavan
From Department of Psychiatry, Government Medical College & Hospital,
Chandigarh, India.
Correspondence to: Dr Jasmin Garg, Department of Psychiatry,
Government Medical College and Hospital, Sector 32, Chandigarh, India.
Email: jasmin.arneja@gmail.com
Received: January 07, 2014;
Initial review: February 06, 2014;
Accepted: May 09, 2014.
|
Objective: To compare the short term efficacy and tolerability of
methylphenidate and atomoxetine in children with Attention deficit
hyperactivity disorder (ADHD).
Design: Open label randomized parallel group
clinical trial.
Setting: Child Guidance Clinic of a tertiary care
hospital of Northern India from October 2010 to June 2012.
Participants: 69 patients (age 6-14 y) with a
diagnosis of ADHD receiving methylphenidate or atomoxetine.
Intervention: Methylphenidate (0.2-1 mg/kg/d) or
atomoxetine (0.5-1.2 mg/kg/d) for eight weeks.
Main outcome measures: Treatment response (>25%
change in baseline Vanderbilt ADHD Diagnostic Parent Rating Scale
(VADPRS); Vanderbilt ADHD Diagnostic Teacher Rating Scale (VADTRS);
Clinical Global Impression Severity Scale (CGI-S) at eight weeks and
adverse effects.
Results: Treatment response was observed in 90.7%
patients from methylphenidate group and 86.2% patients of atomoxetine
group at an average dose of 0.45 mg/kg/d and 0.61 mg/kg/d, respectively.
The patients showed comparable improvement on VADPRS (P=0.500),
VADTRS (P=0.264) and CGI-S (P=0.997). Weight loss was
significantly higher in methylphenidate group (-0.57±0.78 kg; P=0.001),
and heart rate increase was observed at higher rate in atomoxetine group
(7± 9 bpm; P=0.021).
Conclusion: Methylphenidate and atomoxetine are
efficacious in Indian children with ADHD at lesser doses than previously
used. Their efficacy and tolerability are comparable.
Trial Registration No.: CTRI/2011/08/001981
Keywords: ADHD, Adverse events, Efficacy, Treatment dose.
|
|
Attention Deficit Hyperactivity Disorder (ADHD) is
one of the common chronic problems affecting school-age children [1],
and have poor family and peer relations [2]. Without effective
treatment, such children may develop long-term handicaps [3]. According
to current clinical guidelines, psycho-stimulants, especially
methylpheni-date, are considered the first line treatment of ADHD [4,5].
However, methylphenidate is associated with risk of variation in mood
state, motor tics, and abuse potential [6].
Atomoxetine is a nonstimulant that is approved for use in
ADHD as the second line treatment [4,5]. The studies comparing
therapeutic responses to stimulants and atomoxetine in ADHD have been
conducted in Western countries, and have produced conflicting results
[7-13]. The present study was carried out to compare the efficacy and
tolerability of methylphenidate and atomoxetine in Indian children with
ADHD.
Methods
Patients were recruited from those attending the
Child Guidance Clinic of a tertiary care hospital in Northern India from
October 2010 to May 2012. Children (age 6 to 14 years) diagnosed as
ADHD, according to Diagnostic and Statistical Manual of Mental
Disorders-IV-Text Revision [14], and having moderate to severe illness
as assessed by Clinical Global Impressions Severity Scale (CGI-S) [15]
were eligible for inclusion. Patients with history of
non-response or adverse drug reactions to methylphenidate or atomoxetine
in the past, those who had taken any medication for ADHD in past one
month, or those with history of heart disease, seizures, pervasive
developmental disorder, substance abuse, mental retardation or tic
disorder were excluded.
Parents brought the patients to the child guidance
clinic themselves or when referred by school. Before initiating
treatment, electrocardiogram (ECG) was performed for each patient to
rule out any cardiac abnormality.
Written informed consent was obtained from both
parents/guardians and the children. The principles enunciated in the
Declaration of Helsinki [16] and Indian Council of Medical Research [17]
were complied with. Clearance was obtained from the Ethics committee of
the Government Medical College and Hospital, Chandigarh.
The patients were allotted to Group A or Group B by
simple randomization as per computer generated table of random numbers.
Patients in Group A received immediate release tablet Methylphenidate
(once or twice daily) while those in Group B received tablet Atomoxetine
(once or twice daily). The drugs prescribed were from standard
pharmaceutical companies, approved by the drug committee of the
institute. Patients were started on tablet Methylphenidate (immediate
release) 5 mg once a day, or tablet Atomoxetine 10 mg once a day, on
their first visit as per Clinical Practice Guidelines of Indian
Psychiatric Society [18]. Efforts were made to increase the dose of
Methylphenidate up to 1mg/kg/day and of atomoxetine up to 1.2 mg/kg/d
once or twice daily depending upon the response and tolerability. Weekly
increments of 5 mg were tried for both methylphenidate and atomoxetine.
Patients were assessed at baseline and once weekly or fortnightly till 8
weeks. On each visit, improvement in symptoms was assessed by Vanderbilt
ADHD Diagnostic Parent Rating Scale (VADPRS) [19]. Vanderbilt ADHD
Diagnostic Teacher Rating Scale (VADTRS) Proforma [20] was sent through
the parents to be filled up by teachers at baseline and at 8 week.
Teachers were contacted telephonically to obtain information about
patients’ classroom behavior and for clarifying VADTRS. CGI-S was also
used to assess the severity of illness at baseline and last follow up
visit.
The various side effects were noted on each
assessment on the Adverse Events Checklist prepared for the study. It
was a semi-structured check list enlisting all the common side effects
of methylphenidate and atomoxetine. The parents were asked to rate the
severity of each side effects produced in their children as mild,
moderate and severe. Those who reported mild side effects were continued
on the same dose. For those who developed moderate severity of side
effects, dose was reduced. Those who rated any adverse effect to be
severe were taken out of the study after stopping the medication. They
were then managed as per standard treatment protocol of the department.
Heart rate and blood pressure were also recorded at each visit.
Laboratory investigations including complete blood counts, renal
function tests and liver function tests were done at baseline, four
weeks and eight weeks. Primary outcome measures were: improvement in
symptoms as assessed by VADPRS, and percentage of patients who developed
various adverse effects for estimation of tolerability. Secondary
outcome measures were: improvement in symptoms as assessed by VADTRS and
CGI-S; and change in patients’ heart rate, blood pressure, weight and
laboratory investigations for assessment of tolerability.
The sample size was calculated based on data from
previous studies that 70% of patients receiving methylphenidate show
improvement of 25% or more in ADHD rating scale [18]. It was planned to
conclude equivalence of atomoxetine with methylphenidate if 25% or more
improvement is seen in 70 ± 30 % of patients. With a power of 70% and an
alpha of 5%, a sample size of 37 per group was calculated. Keeping in
mind a dropout rate of about 5-10%, it was decided to enroll 40 patients
in each group.
Statistical analysis: Data were analyzed using
SPSS version 16.. Significance level was P <0.05 (two tailed).
Fisher’s exact test and Chi square test were used to compare
categorical variables. Independent sample t-test and paired t-test were
administered for analysis of parametric data. Mann Whitney test and
Wilcoxon signed rank test were used for analysis of CGI-S score as this
parameter was not normally distributed.
Results
Out of 84 children randomized to receive either drug,
17 refused after baseline assessment, and were excluded from analysis.
Of remaining, 33 were in methylphenidate group and 36 were in
atomoxetine group (Fig. 1). In both methylphenidate and
atomoxetine groups, combined type of ADHD was the commonest, followed by
inattention type and hyperactive/impulsive type. The baseline
parameters, including VADPRS total score, VADPRS subscale scores, VADTRS
score and CGI-S score were comparable between the two groups (Table
I).
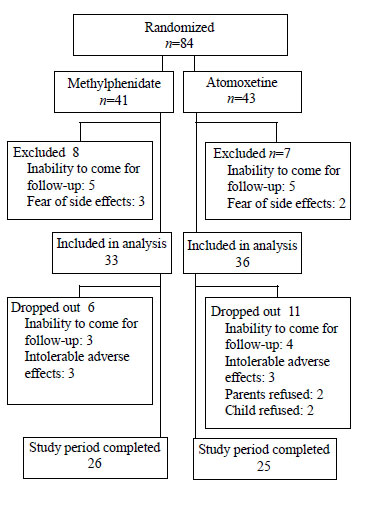 |
|
Fig. 1 Patient flowchart.
|
TABLE I Baseline Characteristics of the Two Groups
|
Variable |
Methylphenidate |
Atomoxetine
|
|
(N=33) |
(N=36) |
|
Age (y)* |
8.47 ± 2.22 |
8.66 ± 2.44 |
|
Weight (kg)* |
28.54 ± 9.45 |
25.26 ± 8.25 |
|
Males, No. (%) |
27 (81.8%) |
29 (80.6%) |
|
Type of ADHD |
|
Inattention |
9 (27.3%) |
6 (16.7%) |
|
Hyperactive/impulsive
|
2 (6.1%) |
4 (11.1%) |
|
Combined |
22 (66.7%) |
26 (72.2%) |
|
Comorbidity |
|
ODD |
15 (45.5%) |
22 (61.1%) |
|
Conduct Disorder |
1 (3%) |
6 (16.7%) |
|
VADPRS* |
|
Total |
51. 18 ± 10.86 |
55.03 ± 14.44 |
|
Inattention |
20.88 ± 3.81 |
19.42 ± 5.50 |
|
Hyperactivity
|
17.85 ± 6.47 |
19.72 ± 5.79 |
|
CGI-S* |
5.03 ± 0.95 |
5.22 ± 0.79 |
|
VADTRS* |
41.11 ± 14..22 |
46.11 ± 14.61 |
|
VADPRS = Vanderbilt
ADHD Diagnostic Parent Rating Scale; CGI-S = Clinical Global
Impression Severity Scale; VADTRS = Vanderbilt ADHD Diagnostic
Teacher Rating Scale; ODD = Oppositional Defiant Disorder; All
values in No.(%) except * values in mean (SD). |
Out of the total 69 recruited patients, 51 (74%)
could be followed up for the eight weeks. Seventeen patients (6 from
methylphenidate group and 11 from atomoxetine group) discontinued the
treatment at some point. There was no significant difference between
mean (SD) baseline VADPRS total score of retained [52.14 (11.99)] and
dropped out [57.29 (14.87)] patients (P = 0.153).
There was significant improvement over 8 weeks in
both methylphenidate and atomoxetine groups when measured on VADPRS
total score, inattention subscale score and hyperactivity subscale
score. The comparative change in VADPRS (total and subscales) scores
from baseline to 8 weeks was not significant (Table II).
TABLE II Change in VADPRS, VADTRS and CGI-S Scores from Baseline to 8 Weeks
|
Variable
|
Methylphenidate |
Atomoxetine |
|
Baseline |
Week
|
Mean |
Intra- |
Base- |
Week |
Mean |
Intra- |
Inter- |
|
|
8 |
difference |
group |
line |
8 |
difference |
group |
group |
|
n = 33 |
n= 26 |
|
P value |
n = 36 |
n = 25 |
|
P value |
P value |
|
VADPRS |
|
Total |
51. 18 (0.86) |
24.69 (10.29) |
-26.69 (1.99) |
<0.001 |
55.03 (14.44) |
23.60 (17.21) |
-29.32 (15.49) |
<0.001 |
0.500 |
|
Inattention |
20.88 (3.81) |
10.375 (5.39) |
-10.00 (4.38) |
<0.001 |
19.42 (5.50) |
7.961 (5.82) |
-11.23 (4.93) |
<0.001 |
0.690 |
|
Hyperactivity
|
17.85 (6.47) |
9.46 (5.11) |
-9.23 (6.14) |
<0.001 |
19.72 (5.79) |
9.00 (6.92) |
-10.20 (7.30) |
<0.001 |
0.610 |
|
CGI-S |
5.03 (0.95) |
2.92 (0.84) |
-2.04 (1.15) |
<0.001 |
5.22 (0.79) |
3.08 (1.55) |
-2.04 (1.37) |
<0.001 |
0.997 |
|
VADTRS |
41.11 (14.22) |
25.29 (9.20) |
-17.2619(10.12)
|
<0.001 |
46.11 (14.61) |
30.42 (14.51) |
-14.10 (9.41) |
<0.001 |
0.264 |
|
VADPRS: Vanderbilt ADHD
Diagnostic Parent Rating Scale; All values in mean (SD). |
With the criteria of 25% reduction in baseline scores
of VADPRS, 90.7% patients from methylphenidate group and 86.2% patients
from atomoxetine group showed improvement. Three (11.5%) patients in
methylphenidate group and five (20%) in atomoxetine group (P=0.465)
showed less than 25% improvement after 2 months in VADPRS scores even
when maximum therapeutic dose was administered.
There was more than 25% improvement in baseline
VADPRS total score from 3rd
week onwards both in methylphenidate and atomoxetine groups when the
mean (SD) dose administered were 11.59 (2.83) mg/day and 14.03 (3.85)
mg/day, respectively. The mean (SD) dose administered at conclusion of
the study when there was maximum efficacy and tolerability was 17.35
(7.52) mg/day (or 0.62mg/kg/day) in the methylphenidate group and 17.46
(7.22) mg/day (or 0.7mg/kg/day) in the atomoxetine group (Web Fig.
1).
According to the adverse effects checklist prepared
for the study, 18 (55%) patients from methylphenidate group and 20 (56%)
from atomoxetine group developed side effects during the course of the
study. The commonest reported adverse effect in both groups was reduced
appetite. There was no significant difference between two groups in the
occurrence of various adverse effects (Table III). Three
patients in each group dropped out due to development of adverse effects
rated as severe by the parents. These side effects were irritability,
fatigue, drowsiness, headache and reduced appetite.
TABLE III Adverse Effects in Methylphenidate and Atomoxetine Groups
|
Adverse effects |
Methylphenidate |
Atomoxetine |
P
|
|
n=32 No. (%) |
n=36 No. (%) |
value |
|
Headache
|
4 (12.5) |
2 (5.6) |
0.410 |
|
Nausea |
1 (3.1) |
1 (2.8) |
1.000 |
|
Vomiting |
1 (3.1) |
1 (2.8) |
1.000 |
|
Decreased appetite |
14 (43.8) |
12 (33.3) |
0.378 |
|
Pain abdomen |
3 (9.4) |
0 (0) |
0.099 |
|
Irritability |
2 (6.3) |
7 (19.4) |
0.157 |
|
Fatigue |
2 (6.3) |
1 (2.8) |
0.598 |
|
Drowsiness |
1 (3.1) |
6 (16.7) |
0.110 |
|
Urinary incontinence |
1 (3.1) |
2 (5.6) |
1.000 |
|
Sadness |
0 (0) |
1 (2.8) |
1.000 |
|
Rash |
1 (3.1) |
0 (0) |
0.471 |
|
Hypersalivation |
1 (3.1) |
0 (0) |
0.471 |
|
Insomnia |
1 (3.1) |
0 (0) |
0.471 |
When assessed on VADTRS and CGI-S, there was
significant improvement over 8 weeks in both methylphenidate and
atomoxetine groups. Teacher’s report was available for 78% of patients.
The change in VADTRS score and CGI-S score from baseline to 8 weeks were
comparable in methylphenidate and atomoxetine groups (Table II).
There was no significant change in the mean (SD) heart rate from
baseline 87 (9)/min to 90 (8)/min at week 8 in methylphenidate group (P=0.312).
However, in atomoxetine group, there was significant increase in heart
rate from baseline 84 (6) to week 8, 92(8)/min with mean difference 7
(9)/min and P =0.021. There was no significant decrease in weight
(in kg) in methylphenidate group from baseline to week 4 [Mean
difference (SD), -0.166 (0.747); P=0.286], but there was
significant decrease at week 8 [-0.576 (0.783); P=0.001]. In the
atomoxetine group, there was no significant weight differences.
There were no significant differences in
hematological and biochemical parameters from baseline to week 4 and
week 8 in either of the groups.
Discussion
The present trial documented that both
methylphenidate and atomoxetine produced statistically significant and
comparable improvements in the symptoms of ADHD, as reported by parents
and teachers. The average dose of both methylphenidate and atomoxetine
which produced significant clinically improvement in the patients of
present study was much lesser than in earlier studies.
Improvements produced by both atomoxetine and
methylphenidate in this study were comparable to that reported in
earlier clinical trials [21]. Absence of teacher’s assessment had been a
potential shortcoming in majority of earlier studies comparing relative
efficacy of the two drugs [7-12]. In our study, the rate of occurrence
of adverse events was comparable to that reported in earlier studies
[7,11,12]. Decreased appetite was the commonest adverse event in both
the groups and methylphenidate led to a little more weight loss than
atomoxetine. The present study had limitations of being an open labelled
study without allocation concealment. Placebo arm was not included due
to ethical considerations. Moreover, lesser number of patients could be
included in the analysis of the study due to the high dropout rate. A
high dropout rate has also been reported in an earlier study from India
[22].
Nonetheless, the present study has important clinical
implications. Equivalent therapeutic efficacy and response rate was
found with lesser doses administered for both the drugs than study
populations in other countries. Atomoxetine was found to be comparable
in efficacy and tolerability methylphenidate in short term. Future
studies with larger sample sizes may be taken up in each subtype of ADHD
with longer duration of follow up in order to document long term effects
of treatment.
Contributors: All authors were involved in concept
and design of study; data collection, analysis and interpretation;
manuscript drafting and its final approval.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Methylphenidate is the first line and
atomoxetine is the second line treatment of ADHD.
What This Study Adds?
• Methylphenidate and atomoxetine have
comparable efficacy in Indian Children with ADHD.
• Dose of methylphenidate and atomoxetine for therapeutic
response seems to be much lower in Indian population than
documented from other settings.
|
References
1. Faraone SV, Sergeant J, Gillberg C, Biederman J.
The worldwide prevalence of ADHD: is it an American condition? World
Psychiatry. 2003;2:104-13.
2. Kidd PM. Attention deficit/hyperactivity disorder
(ADHD) in children: rationale for its integrative management. Altern Med
Rev. 2000;5:402-28.
3. Mannuzza S, Klein RG, Bessler A, Malloy P,
LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J
Psychiatry. 1998;155:493-8.
4. Pliszka S, AACAP Work Group on Quality Issues.
Practice parameter for the assessment and treatment of children and
adolescents with attention-deficit/hyperactivity disorder. J Am Acad
Child Adolesc Psychiatry. 2007;46:894-921.
5. Subcommittee on Attention-Deficit/Hyperactivity
Dis-order; Steering Committee on Quality Improvement and Management,
Wolraich M, Brown L, Brown RT, DuPaul G, et al. ADHD: Clinical
practice guideline for the diagnosis, evaluation, and treatment of
attention-deficit/hyperactivity disorder in children and adolescents.
Pediatrics. 2011;128:1007-22.
6. Greydanus DE, Nazeer A, Patel DR.
Psychopharmacology of ADHD in pediatrics: current advances and issues.
Neuropsychiatr Dis Treat. 2009;5:171-81.
7. Kratochvil CJ, Heiligenstein JH, Dittmann R,
Spencer TJ, Biederman J, Wernicke J, et al. Atomoxetine and
methylphenidate treatment in children with ADHD: a prospective,
randomized, open-label trial. J Am Acad Child Adolesc Psychiatry.
2002;41:776-84.
8. Kemner JE, Starr HL, Ciccone PE, Hooper-Wood CG,
Crockett RS. Outcomes of OROS methylphenidate compared with atomoxetine
in children with ADHD: a multicenter, randomized prospective study. Adv
Ther. 2005;22:498-512.
9. Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K,
Kelsey D. Effects of atomoxetine and methylphenidate on sleep in
children with ADHD. Sleep. 2006;29:1573-85.
10. Prasad S, Harpin V, Poole L, Zeitlin H, Jamdar S,
Puvanendran K, et al. A multi-center, randomized, open-label
study of atomoxetine compared with standard current therapy in UK
children and adolescents with attention deficit/hyperactivity disorder
(ADHD). Curr Med Res Opin. 2007;23:379-94.
11. Wang Y, Zheng Y, Du Y, Song DH, Shin YJ, Cho SC,
et al. Atomoxetine versus methylphenidate in pediatric
outpatients with attention deficit hyperactivity disorder: a randomized,
double-blind comparison trial. Aust N Z J Psychiatry. 2007;41:222-30.
12. Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD,
Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released
methylphenidate for the treatment of attention deficit hyperactivity
disorder: acute comparison and differential response. Am J Psychiatry.
2008;165:721-30.
13. Yildiz O, Sismanlar SG, Memik NC, Karakaya I,
Agaoglu B. Atomoxetine and methylphenidate treatment in children with
ADHD: the efficacy, tolerability and effects on executive functions.
Child Psychiatry Hum Dev. 2011;42:257-69.
14. American Psychiatric Association. Diagnostic and
Stati-stical Manual of Mental Disorder, 4th ed. Text Revision.
Washington DC: American Psychiatric Association; 2000.
15. Busner J, Targum SD. The clinical global
impressions scale: A pplying a research tool in clinical practice.
Psychiatry (Edgmont). 2007;4:28-37.
16. William JR. The Declaration of Helsinki and
public health. Bull World Health Organ. 2008;86:650-2.
17. Indian Council Medical Research. Ethical
Guidelines for Biomedical Research on Human Participants. New Delhi:
Director-General, Indian Council Medical Research; 2006.
18. Gautam S, Batra L, Gaur N, Meena PS. Clinical
practice guidelines for the assessment and treatment of attention
deficit/hyperactivity disorder. In: Gautam S, Avasthi A, editors.
Child and Adolescent Psychiatry Clinical Practice Guidelines for
Psychiatrists in India. Jaipur: Indian Psychiatric Society; 2008. p.
23-42.
19. Wolraich ML, Lambert W, Doffing MA, Bickman L,
Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD
diagnostic parent rating scale in a referred population. J Pediatr
Psychol. 2003;28:559-67.
20. Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A,
Pinnock TY. Obtaining systematic teacher reports of disruptive behavior
disorders utilizing DSM-IV. J Abnorm Child Psychol. 1998;26:141-52.
21. Hanwella R, Senanayake M, de Silva V. Comparative
efficacy and acceptability of methylphenidate and atomoxetine in
treatment of attention deficit hyperactivity disorder in children and
adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176.
22. Sitholey P, Agarwal V, Chamoli S. A preliminary
study of factors affecting adherence to medication in clinic children
with attention-deficit/hyperactivity disorder. Indian J Psychiatry.
2011;53:41-4.
|
|
|
 |
|

