Takotsubo Cardiomyopathy or stress cardiomyopathy is
a heart failure syndrome characterized by transient left ventricular
dysfunction with typical regional wall motion abnormalities [1]. The
wall motion abnormalities are not confined to the vascular distribution
of a single epicardial coronary artery; hence, non-ischemic mechanisms
are considered responsible. Initially described in adult women with
emotional stress as ‘broken-heart syndrome’, it was subsequently
recognized in both males and females. Takotsubo cardiomyopathy has been
reported rarely in children. We describe a case of Takotsubo
cardiomyopathy associated with sepsis in a child.
A 10-year-old boy presented with complaints of
high-grade fever since four days and fast breathing and poor oral intake
since one day. On examination, he had tachycardia, hypotension,
respiratory distress, generalized edema and hepatomegaly. High-flow
oxygen, intravenous fluids and inotropes were started (noradrenaline
followed by dobutamine). Laboratory investi-gations revealed a
C-reactive protein of 101 mg/L. As clinical and laboratory features were
suggestive of severe sepsis, intravenous antibiotics were started. Since
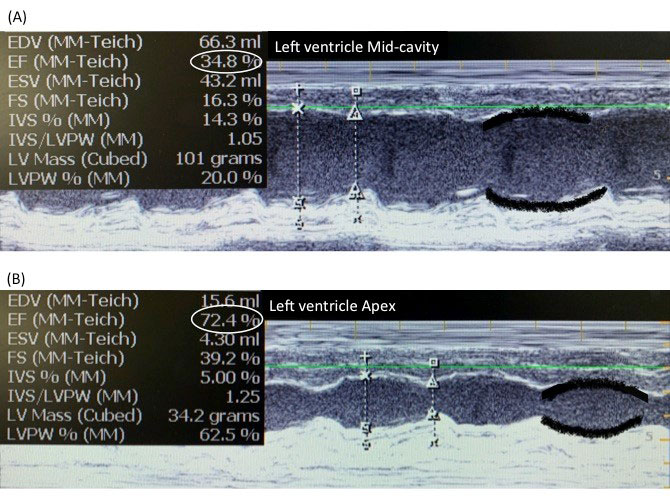
hypotension persisted, 2D echocardiogram was done, which showed
mid-ventricular regional wall motion abnormality (Fig. 1a), with
normal contractility of cardiac apex (Fig. 1b) and mild
mitral regurgitation. ECG showed mild ST elevation in lead V2. Troponin
level was mildly elevated (16.4 ng/L), and B-type natriuretic peptide
(BNP) markedly elevated (15725 pg/mL). In view of a diagnosis of
Takotsubo cardiomyopathy, noradrenaline was tapered and diuretic drugs
were prescribed. A repeat echocardiogram after three days showed
normalization of left ventricular function and resolution of mitral
regurgitation. Repeat ECG showed T wave inversion in V2. Work-up for
persistence of fever spikes and thrombocytopenia revealed negative
dengue serology, and positive result for OX K antigen on Weil Felix test
The child showed improvement on oral Doxycycline and was discharged
without any cardiac medications by ninth day of hospitalization.
 |
|
Fig. 1 M-mode Echocardiogram of Left
ventricle (LV). (a)Poor contractility of LVMid-cavity with
Ejection fraction (EF) of 34%. The black lines marked along
anterior and posterior LV walls show poor excursion with time,
(b) Normal contractility of LV Apex with EF: 72%. The black
lines highlight excellent approximation during systole.
|
Takotsubo cardiomyopathy is a reversible heart
failure syndrome. It derives its name from the shape of the left
ventricle in the typical apical-ballooning form, which resembles a
Japanese octopus-trap. Four common morphological variants have been
described [1]. In younger patients, a high proportion of non-apical
anatomical variants are seen [2]. Our case was of the mid-ventricular
type, with hypokinesia of the mid-ventricle and sparing of the apex.
Distinguishing Takotsubo syndrome from acute
infective myocarditis can be challenging. However, involvement in
Takotsubo cardiomyopathy shows typical regional pattern whereas in
myocarditis it tends to be diffuse, and it shows a low or moderate
troponin rise while there is frequently a significant rise in troponin
in myocarditis [1]. The differential diagnosis includes sepsis-induced
cardiomyopathy, but in the latter too, there is global involvement and
enlargement of the ventricle, as opposed to regional affection in the
former [3].
Regional wall motion abnormality of Takotsubo cardio-myopathy
requires differentiation from acute coronary synd-rome, a less common
entity in children. Characteristically laboratory evaluation shows an
extremely elevated BNP and mild troponin elevation. In contrast, in
coronary ischemia, the degree of BNP elevation is typically lesser than
in Takotsubo cardiomyopathy, troponin is significantly elevated and ECG
and echocardiographic wall motion abnormalities are confined to the
vascular distribution of epicardial coronary arteries [4]. ECG in
Takotsubo cardiomyopathy may show non-specific ST changes, with later
development of T wave inversion, as seen in our case, or even QTc
prolongation [1]. Cardiac magnetic resonance during the acute phase may
help differentiate Takotsubo cardiomyopathy from both, myocarditis as
well as acute myocardial infarction [2].
Sepsis has been widely described in adults as a cause
for Takotsubo cardiomyopathy, with a causative organism identifiable in
culture in up to 50% of admissions [5]. Our search of English language
scientific literature did not reveal any report of this disorder in
pediatric sepsis following scrub typhus infection. There is a
possibility that scrub typhus as a cause is either unrecognized or
under-reported.
Management of this disorder is unique in that
catecho-lamines are incriminated in the pathophysiology and hence, must
be avoided. Beta-blockers and ACE inhibitors may be used. Mechanical
support such as extracorporeal membrane oxygenation may be required. For
inotropic support, Levosimendan is considered safe, and has been
reported to be effective specifically in sepsis-associated Takotsubo
cardiomyopathy [6].
The long-term prognosis is favorable, but it is now
recognized that upto 50% can have acute complications with a mortality
rate of 4-5 % [1].
To conclude, Takotsubo cardiomyopathy may be
identified by the typical echocardiographic appearance and laboratory
features, and entails specific management considerations. Recognition of
the entity is of importance because of the unique management approach
that it entails
1. Lyon AR, Bossone E, Schneider B, et al. Current
State of Knowledge on Takotsubo Syndrome: A Position Statement from the
Task Force on Takotsubo Syndrome of the Heart Failure Association of the
European Society of Cardiology. Eur J Heart Fail. 2016;18:8-27.
2. Urbinati A, Pellicori P, Guerra F, Capucci A,
Clark AL. Takotsubo syndrome in the paediatric population a case report
and a systematic review. J Cardiovasc Med. 2017;18: 262-7.
3. Sato R, Nasu M. A review of sepsis-induced
cardiomyopathy. J Intensive Care. 2015;3:48.
4. Gopalakrishnan P, Zaidi R, Sardar MR. Takotsubo
cardiomyopathy: Pathophysiology and role of cardiac biomarkers in
differential diagnosis. World J Cardiol. 2017;9:723-30.
5. Vallabhajosyula S, Deshmukh A, Kashani K, Prasad
A, Sakhuja A. Takotsubo cardiomyopathy in severe sepsis: Nationwide
trends, predictors, and outcomes. J Am Heart Assoc. 2018;7:e009160.
6. Karvouniaris M, Papanikolaou J, Makris D,
Zakynthinos E. Sepsis-associated takotsubo cardiomyopathy can be
reversed with levosimendan. Am J Emerg Med. 2012;5:832.e5-832.e7.

