The pertussis vaccine is
effective in preventing Bordetella pertussis
infection and death, and the risk is high in young
infants who do not receive the vaccine [1]. B.
pertussis infection in siblings is considered a
common route of transmission to young infants [2].
Currently, three brands of DPT-IPV (acellular
pertussis, diphtheria and tetanus toxoids, and
inactivated polio combined) are used in Japan. All
contain pertussis toxin and filamentous
hemagglutinin (6-23.5 and 23.5-51.5 µg/0.5 mL,
respectively), and one contains additional pertactin
and fimbriae (5 and 1 µg/0.5 mL, respectively) [3].
Children receive a total of four doses of DPT-IPV:
three primary doses at the ages of 3, 4, and 5
months, and one booster dose at 18 to 23 months as a
national routine vaccination. In 2018, vaccine
coverage for the four doses was 95.0%, 95.7%, 96.2%,
and 96.2%, respectively [4]. The preschool pertussis
vaccination booster is used in some Asian countries
like India, but not in Japan [5]; even though Japan
has one of the highest primary pertussis vaccination
rates in the world [6]. We, herein present data from
an outbreak of pertussis, which occurred mainly in
lower-grade school children without preschool
vaccination boosters.
A retrospective chart-based study
was conducted on patients who visited the Saiwai
Pediatric Clinic, Tokyo, Japan, with persistent
cough. Patients were examined by board-certified
pediatricians for suspected pertussis and received a
laboratory diagnosis between August and September,
2018. In accordance with the Pediatric Respiratory
Infection Practice Guidelines in Japan [7],
diagnostic tests for pertussis were defined as
positive by either nasal swab loop-mediated
isothermal amplification (LAMP) or anti-pertussis
IgM/IgA in sera. The positive and negative
predictive rates of LAMP are 100% and 97%,
respectively (Loopamp Pertussis Detecting Reagents
D; Eiken Chemical Corporation). The sensitivity of
anti-pertussis IgM and IgA are 29-56% and 25-44%,
respectively and the specificities are 93% and 99%,
respectively (Novagnost Bordetella pertussis
IgM/IgA; Siemens Healthcare Diagnostics KK). The
patient background (sex, age, and vaccination
history), and diagnosis method were collected.
Statistical analyses included a
bar graph review and Fisher exact test of
age-distribution comparisons. We used SPSS
Statistics 25 (IBM Corp.) and BellCurve for Excel
for Windows (Social Survey Research Information Co.
Ltd.) software programs.
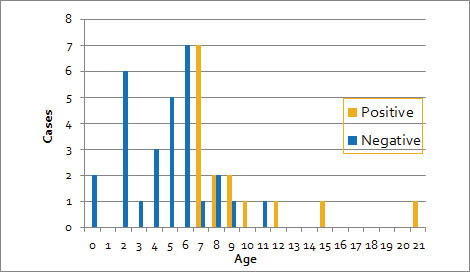
Of the 44 patients (age
distribution: 0-21 years, median: 6 years), data of
15 patients who were diagnosed with
laboratory-confirmed pertussis (age: 7-21 years,
median: 8 years) and 29 patients who were pertussis-negative
(age: 0-11 years, median: 5 years) were compared.
All patients (n=15) who were pertussis-positive
but only 17% (n=7) of patients who were
pertussis-negative were elementary school age and
older (P< 0.001) (Fig. 1). All 16
preschool children were negative. Excluding
serodiagnosis cases (3 positive cases, 1 negative
case), a significant difference in age distribution
(P<0.001) was also observed. When a 2 × 2
table was prepared with 7 years of age as the
cut-off value, the sensitivity, specificity,
positive predictive rate, and negative predictive
rates were 100%, 83%, 75%, and 100%, respectively.
 |
|
Fig. 1 Pertussis
test results and age distribution.
|
None of the 44 patients had a
history of preschool vaccination booster at around
5 years of age. Of 15 children who were positive, 14
patients had received four routine vaccinations and
the booster history was uncertain in 1 patient. Of
29 children who were negative, 26 patients had
received four routine vaccinations, but two
patients who were a few months old were vaccinated 0
and 2 times, and the booster history was uncertain
in another patient.
The absence of pertussis in
preschool children and the presence of pertussis in
lower-grade schoolchildren suggest the need of
additional preschool vaccinations. It has been
reported that the prevalence of anti-pertussis toxin
titer in individuals aged 4 to 7 years declines to
26-38% even among regular vaccines [8]. During our
research period, the Japanese Society of Pediatrics
began to recommend that preschool children aged 5 to
6 years receive a DPT vaccination, but this is on a
voluntary basis [9].
This report covers a limited
number of cases in a single institution, and the
question remains of whether the data on sensitivity
and specificity for pertussis at age 7 years or
older can be generalized. However, the all-Japanese
pertussis cases reported indicate that over 60% of
cases are between the ages of 6 and 15 years,
peaking at the age of 7 years [10], which is
consistent with the age distribution reported
herein.
We experienced a pertussis
epidemic in elementary school-age children, all of
whom had been immunized with at least three doses of
primary DPT-IPV immunization. We believe that
popularizing the preschool pertussis vaccination is
important in order to eliminate the infection source
for young infants. Consideration should be given to
routine preschool pertussis vaccination boosters in
Japan if larger community-based studies confirm our
findings.
Ethics approval: Keio
University School of Medicine Ethical Committee,
Tokyo, Japan; No. 20190205, dated December 26, 2019.
Contributors: TK, MS, AM, TT:
designed the study; TK, MS, AM: collected and
analyzed data: TK, MS, AM, TT: wrote the manuscript;
AM,TT: critically reviewed the manuscript. All
authors read and approved the final manuscript.
Funding: None; Competing
interests: None stated.
1. Kavitha TK, Samprathi M,
Jayashree M, et al. Clinical profile of critical
pertussis in children at a pediatric
intensive care unit in Northern India. Indian
Pediatr. 2020; 57:228-31.
2. Skoff TH, Kenyon C, Cocoros N,
et al. Sources of infant pertussis infection in the
United States. Pediatrics. 2015; 136:635-41.
3. Nakayama T. Current status of
pertussis and the need for vaccines. Pediatrics of
Japan. 2019;60:1137-44 (in Japanese).
4. Ministry of Health, Labour and
Welfare. Implementation of routine immunizations.
Accessed September 20, 2020. Available from:
http://www.mhlw.go.jp/topics/bcg/other/5.html
5. World Health Organization. WHO
vaccine-preventable diseases: monitoring system.
2020 global summary. Accessed September 20, 2020.
Available from:
https://apps.who.int/immunization_ monitoring/globalsummary/
6. UNICEF. Immunization coverage
estimates data visualization. Accessed September 20,
2020. Available from:
https://data.unicef.org/resources/immunization-coverage-estimates-data-visualization
7. Ouchi K, Okada K, Kurosaki T.
Guidelines for the management of respiratory
infectious diseases in children in Japan.
2017;53:236-40.
8. National Institute of
Infectious Diseases. Seroprevalence of pertussis in
2013, Japan. 2013. Accessed September 20, 2020.
Available from:
https://www.niid.go.jp/niid/images/iasr/2017/02/444tef05.gif
9. Japan Pediatric Society.
Vaccination Schedule Recommended by the Japan
Pediatric Society. 2018. Accessed September 20,
2020. Available from:http://www.jpeds.
or.jp/uploads/files/20180801_JPS%20Schedule%20
English.pdf
10. National Institute of
Infectious Diseases. Number of notified pertussis
cases by age and immunization status week 1 to week
48 of 2018, Japan (n=9674). 2018. Accessed
September 20, 2020. Available from:
https://www.niid.go.jp/niid/images/iasr/2019/01/467tef04.gif

