|
A pesticide is a substance or
mixture of substances intended to prevent, destroy, repel, or lessen the
damage caused by a pest[1]. Most pesticides are poisonous to humans and
if released into the environment, have significant adverse ecological
effects. Endosulfan is one such compound extensively used in agriculture
around the world, belonging to the organochlorine group of pesticides.
In India, more endosulfan is produced than any other
pesticide except mancozeb and monocrotophos [2]. The advantage of
endosulfan over other safer pesticides is its low cost, easy
availability and extreme efficacy against the Tea mosquito bug (H.
antonii), a dreaded pest in cashew and cash crop plantations. The US
Environmental Protection Agency and the European Union classifies
endosulfan under Category 1b – highly hazardous. Aerial spraying of
endosulfan was banned by court order in 2001 [3]. However, there are
reports of the continued use of endosulfan in areas where cashew and
other cash crop plantations are aplenty.
A number of studies have highlighted various adverse
effects of Endosulfan but its role in leukemogenesis has not been
conclusively established [4]. Pandey, et al. [5] demonstrated the
genotoxic potential of endosulfan by demonstrating DNA damage following
experimental exposure in fish. Being a lipophilic compound, we
postulated that endosulfan is likely to concentrate in the fatty bone
marrow, affect the progenitor cells and subsequently adversely influence
the maturation of all hemopoietic cell lines. The study by Olea, et
al. [6] revealed that isomers and metabolites of endosulfan were
present in the fatty tissues of 30-40% of hospitalized children in the
agricultural regions of Spain.
We undertook this study to assess if children with
hematological malignancies have increased amounts of endosulfan in their
bone marrow in comparison to matched controls without hematological
malignancies, and further determine if these children resided in area
where Endosulfan had been or is being used.
Methods
This was a case control study, involving children in
the age group of 1-15 years conducted in the constituent hospitals of a
medical college in Dakshina Kannada district of Karnataka, over an 18
month period from September 2006 to March 2008.
26 cases of proven hematological malignancy and 26
age matched controls who presented serially to our hospital during the
study were recruited. Case criteria being children in the age group 1-15
years, with proven hematological malignancies by bone marrow study.
Controls were age matched children with proven absence of hematological
malignancies by bone marrow aspiration analysis, but who required the
bone marrow aspiration for diagnosis. The study period was 18 months.
The patients were interviewed to record relevant
clinical history and clinical findings in a structured proforma. Details
about family and other pre-existing illness were elicited with special
reference to other important diseases reported in the area where the
patients usually resided. Parents were explained the objective of the
study and after written consent of one of the parents, the children were
examined and a bone marrow aspiration done under short dissociative
anaesthesia. In addition to relevant studies for diagnosis, Endosulfan
residues in the bone marrow were estimated. In those patients where
Endosulfan was detected, we determined their geographical location to
determine the source of exposure. The Institutional Ethics Committee
approved this study.
Methodology for Endosulfan Analysis
Detection of low levels of endosulfan involves
extraction of samples with organic solvents, a cleanup step to remove
lipids and other materials that may interfere with analysis, High
resolution gas chromatography (HRGC) to separate endosulfan from other
compounds in the extract, and confirmation of endosulfan by Electron
capture detector (ECD) or Mass spectroscopy (MS).
Extraction of endosulfan from bone marrow: 150µL
of ethyl acetate was added to 250µL bone marrow sample followed by 600µL
of cold 60% H2SO4.
The sample was vortexed for 1 minute and 3 mL of n-hexane-acetone
mixture (90:10, v/v) was added for drug extraction. The mixture was
vortexed again and centrifuged at –10° C at 2500 × g for 25
minutes. The organic phase was collected and evaporated to dryness in a
water bath at 50° C under a gentle stream of nitrogen. The dried residue
was reconstituted with 250µL of ethyl acetate and vortexed for 1 minute.
The supernatant was transferred entirely into a 250µL vial-insert and a
volume of 2µL was injected into the gas chromatography mass spectrometry
(GC-MS) system.
Validity of the method was studied and the method was
found to be precise and accurate with a linearity range from 10 to
100ng/mL (r2 >0.998). The
quantification limit (LOQ) was found to be 10 ng/mL. The levels of all
three forms of endosulfan i.e. alpha, beta and endosulfan sulfate (a
metabolite) were estimated.
Instrument conditions: The GC-MS system consisted
of a Shimadzu QP 5000 mass spectrometer and a Shimadzu GC 17A gas
chromatograph equipped with an AOC 14000 autosampler and a GCMS solution
(version 1.10) software (Shimadzu, Courtaboeuf, France). The capillary
column used was a DB-5 ms (30 m × 0.25 mm (id), 0.25 µm film thickness.
The injector was set at a temperature of 240° C and used in splitless
mode. The carrier gas was grade N55 helium and its flow rate was 2.1 mL/min.
Column initial temperature was 60° C held for 2 min, then increased at
10şC min-1 to 280° C.
interface was set at 290°C. Selective ion mode (SIM) was used for
quantification. The specific fragment ions in SIM mode were: alpha-endosulfan:
195, 237, 339; beta-endosulfan 195, 229, 341; endosulfan sulfate: 229,
272,385. Retention times were alpha-endosulfan: 20.3 min, beta-endosulfan:
21.5 min and endosulfan sulfate: 22.5 min. Ionization was performed in
electron impact mode at 70 eV. The minimum detection limits of
α–endosulfan,
β endosulfan
and endosulfan sulphate were 10ng/mL.
Standard reference material:
a–endosulfan:
detection purity - 98.5%; b-endosulfan:
detection purity - 98%. The spectrochromatograms of the various samples
showed specific molecular ion peaks when detectable quantities of the
Endosulfan isomers were noted. The peak obtained in the study subjects
was compared with those of the controls. Recovery tests were performed
to check the efficiency of the extraction procedure by spiking known
quantities of standard endosulfan to the samples and determining the
quantities by the method developed.
The data obtained was entered in an appropriate
format and analyzed using SPSS version 11.0. Chi-square test was applied
on the data and a P-value of less than 0.05 was taken to be
statistically significant. The Odds ratio was calculated on appropriate
data to determine the disease risk associated with the exposure.
Results
A total of 52 children were enrolled for the study of
which 26 were study cases (23 acute lymphoblastic leukemia, 3 myeloid
leukemia) and the remaining controls. In the control group, 50% cases
were immune thrombo-cytopenic purpura, 23% refractory anemias, 15% had
pyrexia of unknown origin, and 12% had atypical juvenile rheumatoid
arthritis. Both groups were comparable statistically (Table
I). A total of 36 children in the study hailed from sprayed
areas of which 21 (58.3%) and 15 (41.7%) belonged to the study and
control groups, respectively. Parental occupation revealed no relation
on analysis of either mother’s or father’s occupation; but was
significant when considering fathers occupation, wherein, a significant
number of fathers in the study group were found to be farmers or in
occupations involving exposure to pesticides, as compared to the control
group.
TABLE I Socio-Demographic Profile of Study Subjects
|
Cases
|
Controls |
P
|
|
(n=
26) |
(n=26) |
value
|
|
No
(%) |
No
(%) |
|
| Age (Yrs) |
| < 5
|
13 (50) |
7 (26.9) |
0.19 |
| 5 to 10 |
9 (34.6) |
11 (42.3) |
|
| >10 |
4 (15.4) |
8 (30.8) |
|
| Male Sex |
15 (57.7) |
14 (53.8) |
0.78 |
| Socio economic status |
| Upper
|
1 (3.8) |
1 (3.8) |
0.33 |
| Upper
Middle |
5 (19.2) |
1 (3.8) |
|
| Lower
Middle |
4 (15.4) |
9 (34.6) |
|
| Upper
Lower |
14 (53.8) |
13 (50) |
|
| Lower |
2 (7.7) |
2 (7.7) |
|
| Place of Residence |
|
|
|
| Dakshina
Kannada |
15 (57.6) |
11 (42.3) |
|
| (including
Mangalore)* |
| Kasargod* |
6 (23.1) |
4 (15.4) |
|
| Kannur |
2 (7.7) |
5 (19.2) |
|
| Others |
3 (11.5) |
6 (23.0) |
|
| Father’s Occupation |
| Agriculture |
6 (23.1) |
4 (15.2) |
|
| Business |
8 (30.7) |
12 (45.6) |
|
| Coolie |
11 (42.3) |
7 (26.6) |
|
| Others |
1 (3.8) |
10 (38.4) |
|
| Mother’s Occupation |
| Housewife |
17 (65.4) |
19 (73.1) |
|
| Beedi
roller |
8 (30.8) |
5 (19.2) |
|
| Others |
1 (3.8) |
2 (7.7) |
|
*Areas sprayed with endosulfan.
Endosulfan estimation in the bone marrow revealed
that of the seven children (6 study, 1 control) who had detectable
levels of alpha and beta endosulfan, six were also found to be positive
for endosulfan sulfate, a degradation metabolic product of both alpha
and beta isomers. All 6 of them belonged to study group. The odds ratio
was 7.5 (95% CI: 1.34, 176.43), indicating a 7.5 times higher risk of
developing hematological malignancy in children with detectable
endosulfan levels compared to those with undetectable endosulfan in the
bone marrow. Fig. 1 shows the peak detectable
concentration of alpha,beta and sulphate isomers of endosulfan in a
positive bone marrow sample.
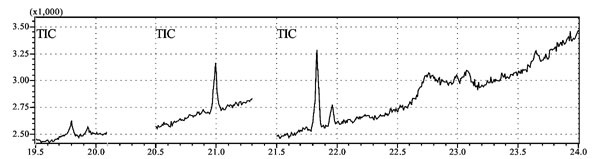 |
|
Fig.1 Endosulfan isomers in
chromatograph of a positive bone marrow sample.
|
All the children who had raised endosulfan levels in
the bone marrow originated from the areas, which were or are still
exposed to endosulfan.
Discussion
Exposure to endosulphan in the environment results in
bioaccumulation in humans and other animals, concentrating particularly
in fatty tissues [7].
Zahm and Ward [8] found that children who live on or
whose parents work on a farm, had higher levels of pesticides in the
immediate environment of their homes, compared to children who do not.
Shu, et al. [9] reported that those with acute lymphoblastic
leukemia (ALL) were 3-5 times more likely to have mothers who had been
occupationally exposed to pesticides during pregnancy, compared to
healthy children. Infante- Rivard, et al. [10] found that
children with ALL were 3-9 times as likely to have parents who were
exposed to pesticides during pregnancy or lactation. In a more recent
study, Ma, et al. [11] found that the use of professional pest
control services at home, at any time from 1 year before birth to 3
years after, was also associated with a 2.8 fold increase in the
likelihood of developing childhood leukemia. A study conducted by the
Children Cancer Study Group found that children with acute
non-lymphocytic leukemia (ANLL) were more than 2.5 times as likely as
children without the disease to have fathers who had used pesticides
occupationally for more than 1000 days [12]. However, in this study, in
both groups, a number of fathers were found to be residing away from
home due to demands of the job, whereby the chance of exposure due to
father’s occupation was minimal.
We found seven children with detectable bone marrow
endosulfan levels and all seven resided in areas which were and are
reportedly still exposed to endosulfan. These children had more than
10ng/mL of endosulfan in their bone marrow, as the lowest detectable
limit of endosulfan by our methods was 10ng/mL. These seven children may
represent only the tip of the iceberg, as we have no knowledge of the
baseline bone marrow endosulfan levels in apparently normal children nor
do we have age based normograms to this effect. Six of these children
were also found to be positive for endosulfan sulfate, a degradation
metabolic product of both alpha and beta isomers, which is more
persistent in the body than the parent compound and indicates chronic
exposure to the pesticide [13]. In a study done by the National
Institute of Occupational Health in 2002, Kasargod district (North
Kerala), it was found that the serum endosulfan levels were
significantly higher in the study population (from areas exposed to
endosulfan) as compared to the reference group (hailing from areas which
had never been exposed). Endosulfan residues were found in 85% and 78%
of female and male subjects, respectively hailing from the study area
compared to 34% and 29% of female and male subjects in the reference
group [14]. Cerrillo, et al. [15] also found evidence of
significant accumulation of endosulfan in humans exposed to it in South
Spain. There are no earlier studies in literature to support our
finding. Although there are no human studies relating to the
carcinogenicity of endosulfan to date, a study done by National Cancer
Institute, USA in 1978 had shown that endosulfan can induce
lymphosarcomas and neoplasms of the reproductive system in rats. Reuber,
et al. [16] found endosulfan to be carcinogenic in the liver of
female mice.
This study does not in any way proves that endosulfan
is a cause of leukemia, rather it affirms the fact that Endosulfan, a
lipophilic compound, has the potential to accumulate in the bone marrow,
the site of fundamental cell biological processes that control cell
differentiation and maturation and can hence affect the outcome and
functioning of various organ systems subsequently. To the best of our
knowledge, this study is the first of its kind where bone marrow samples
of children has been subjected to analysis for pesticide levels and this
may serve as the basis for future larger studies. The small sample size,
other confounding factors like genetic susceptibility, exposure to other
kinds of pesticides and carcinogens and their effects, are the
limitations of the study.
Greater awareness of the toxic effects and improper
use of pesticides needs to be created among the public. Siblings of
children with leukemia may need to be screened for pesticide levels to
prevent chronic long term exposure in the future. Large scale,
multidisciplinary, prospective studies along with cytogenetic analysis
are recommended to evaluate the leukemogenic potential of endosulfan.
Acknowledgments: Dr Goutham Shenoy and Mr Sriram
Pathak of the Department of Pharmaceutical Chemistry, College of
Pharmaceutical Sciences, Manipal for performing the chemical analysis on
the specimen.
Contributors: ATK: conceived, designed and
authored the first manuscript of the study and subsequently revised it
for important intellectual content. He is the guarantor of the study;
ATK, AC, KSA: recruited the subjects and collected the data;
Hematological investigations were carried out by AR; ATK, AC, KSA and
AR: wrote the final manuscript with inputs from RW who also monitored
the progress of the study. All authors reviewed and approved the final
manuscript.
Funding: Manipal University, Manipal;
Competing interests: None stated.
|
What Is Already Known?
• Endosulfan is a pesticide with a wide
variety of adverse effects on all living beings.
What This Study Adds?
• Endosulfan was detected in the bone marrow of children with
hematologic malignancies.
|
References
1. About Pesticides. U.S Environmental Protection
Agency. Available from: http://www.epa.gov/opp00001/about/. Accessed
June 10, 2010.
2. Saiyed H, Dewan A, Bhatnagar V, Shemov U, Shenoy
R, Rajmohan H, et al. Effect of endosulfan on the male
reproductive development. Environ Health Perspective. 2003;111:1958-62.
3. Devakumar C. Endosulfan aerial spray controversy
in Kerala. Pesticide Research Journal. 2002; 14:343-44.
4. California Pesticide Use Reporting Data,
California Department of Pesticide Regulation, 1997-2007, cited in
Pesticide use in California, www.pesticideinfo.org.
5. Pandey S, Nagpure NS, Kumar R, Sharma S,
Srivastava SK, Verma MS. Genotoxicity evaluation of acute doses of
endosulfan to freshwater teleost Channa punctatus (Bloch) by
alkaline single-cell gel electrophoresis. Ecotoxicology and
Environmental Safety. 2006;65:56-61.
6. Olea N, Olea - Serrano F, Lardelli- Claret P,
Rivas A, Barba-Navarro A. Inadvertent exposure to xenoestrogens in
children. Toxicol Ind Health. 1999;15:151-8.
7. NIOSH Pocket Guide to Chemical Hazards. US Dept of
Health and Human Services- National Institute for Occupational Safety
and Health 2004.
8. Zahm SH, Ward M, Blair A. Pesticides and Cancer.
Occup Med. 1997;12:269-89.
9. Shu XO, Gao YT, Brincton L A, Linet M S, Tu J T,
Zheng W, et al. A population based case control study of
childhood leukemia in Shangai. Cancer. 1988;62:635-44.
10. Infante Rivard C, Labuda D, Krajinovic M, Sinnett
D. Risk of childhood leukemia associated with exposure to pesticides and
with gene polymorphisms. Epidemiology. 1999;10:481-87.
11. Max Buffler P A, Gunier RB, Dahl G. Critical
windows of exposure to household pesticides and risk of childhood
leukemia. Environ Health Perspectives. 2002;110: 955-60.
12. Buckley JD, Robison LL, Swotinsky R, Garabrant
DH, Le Beau M, Manchester P, et al. Occupational exposures of
parents of children with acute nonlymphocytic leukemia: A report from
the Children’s Cancer Study Group. Cancer Research. 1989;49: 4030-7.
13. Jayashree R, Vasudevan N. Effect of tween 80
added to the soil on the degradation of Endosulfan by Pseudomonas
aeruginosa. Int J Environ Sci Tech. 2007; 4:203-10.
14. Final report of the investigation of unusual
illnesses allegedly produced by Endosulfan exposure in Padre village of
Kasargod district (N Kerala). National Institute of Occupational Health.
2002: 43.
15. Cerrillo I, Granada A, Lopez Espinosa MJ, Olmos
B, Jimenez M, Cano A, et al. Endosulfan and its metabolites in
fertile women, placenta, cord blood and human milk. Environ Res.
2005;98:233-9.
16. Reuber MD. The role of toxicity in the carcinogenicity of
Endosulfan. Sci Total Environ. 1981; 20: 23-47.
|

