Pathogenesis
These organisms grow in
alimentary canal of arthropods. Arthropods maintain
the infection naturally by either transovarial
transmission (passage of the organism from infected
arthropods to their progeny seen in spotted fever
group and scrub typhus) wherein arthropods act as
vector as well as reservoir; or without transovarial
transmission seen in typhus fever group, wherein
arthropods act only as vector. Man is an accidental
host except for louse borne epidemic typhus caused
by Rickettsia prowazekii. Transmission to
humans occurs by infected arthropod vector or
exposure to infected animal reservoir host. Vector
to human transmission occur as vector defaecate
while feeding (flea feeding reflex) so that faces
contaminate pruritic bite wounds (seen with typhus
fever group) or primarily by bite, where
regurgitation of infected saliva occurs during
feeding (seen with spotted fever group and scrub
typhus).They are not transmissible directly from
person to person except by blood transfusion or
organ transplantation(10).
Pathology
These organisms after entering
human body, multiply locally and enter the
bloodstream. Then they invade their target cells,
which are vascular endothelium, reticuloendothelial
cells and in case of Ehrlichiosis and Anaplasmosis,
blood cells. Once inside host cells, organisms
multiply and accumulate in large numbers before
lysing the cell (in case of typhus group) or they
escape from cell, damaging its membrane and causing
influx of water (in case of spotted fever group).
Unlike rickettsiae in the spotted fever group, which
can survive and replicate for several days after the
death of their host cells, rickettsiae of the typhus
group die rapidly after killing their host
cells(11).
Vasculitis is the basic
pathogenetic mechanism. Vasculitis is responsible
for skin rash, microvascular leakage, edema, tissue
hypoperfusion and end-organ ischemic injury.
Formation of thrombi can lead to tissue infarction
and hemorrhagic necrosis. Inflammation and vascular
leakage leads to interstitial pneumonitis,
noncardiogenic pulmonary edema, cerebral edema and
meningoencephalitis. Infection of endothelial cells
also induces procoagulant activity that promotes
coagulation factor consumption, platelet adhesion
and leucocyte emigration and may result in clinical
syndrome similar to disseminated intravascular
coagulation(12).
Clinical Features
Early signs and symptoms of these
infections are nonspecific and mimic benign viral
illnesses, making diagnosis more difficult(13).
Symptomato-logy may vary from mild to severe. Unless
there is a high index of suspicion, it is likely to
be missed as the clinical presentation may mimic
other common infections in the tropics(14).
Incubation period of various rickettsial infections
varies between 2-21 days. Clinical manifestations of
rickettsial infections are detailed herein.
Fever: Fever of
undetermined origin is the most frequent
presentation of rickettsial disease. Fever is
usually abrupt onset, high grade, sometimes with
chills, occasionally with morning remissions and
associated with headache and myalgia. Diagnosis of
rickettsial disease should always be considered in
patients with acute febrile illness accompanied with
headache and myalgia, particularly in endemic areas
with history of tick exposure or contact with dogs.
In one study, 24% among 180 children (less than 14
years age) admitted with acute febrile illness in
whom other common causes for fever were excluded,
were clinically and serologically confirmed to have
scrub typhus or other rickettsial infections. Scrub
typhus formed the largest group (62.8%) followed by
spotted fever (32.6%) and endemic typhus fever
(4.7%)(15).
Headache and Myalgia:
Severe frontal headache and generalised myalgia
specially in muscles of the lumber region, thigh and
calf is seen in variable proportion of cases.
Headache is noted less frequently in young children
than in adults, but when it occurs, it is often
intractable to therapy(16).
Rash: Though rash is
considered as hallmark of rickettsial disease, it is
neither seen at presentation nor in all the
patients(17,18). Thus it should be remembered that
spotted fevers could be spotless too! Rash usually
becomes apparent after 3-5 days of onset of
symptoms. Initially rash is in the form of pink,
blanching, discrete macules which subsequently
becomes maculopapular, petechial or hemorrhagic (Fig.1).
Sometimes palpable purpura (typical of vasculitis)
is seen. Occasionally petechiae enlarge to
ecchymosis and gangrenous patches may develop.
Rarely gangrene of digits, earlobes, scrotum, nose
or limbs may occur secondary to vasculitis and
thrombosis. Distribution of rash is initially near
ankles, lower legs and wrists. Thereafter rash
spreads centripetally to involve whole body.
Presence of rash on palms and soles, considered so
typical of rickettsial disease, can be seen in other
diseases like infective endocarditis, syphilis,
meningococcemia, enteroviral diseases and adverse
drug reactions. The rash of typhus group
rickettsioses is quite atypical, initially appearing
on trunk, spreading centrifugally and usually
sparing palms and soles.
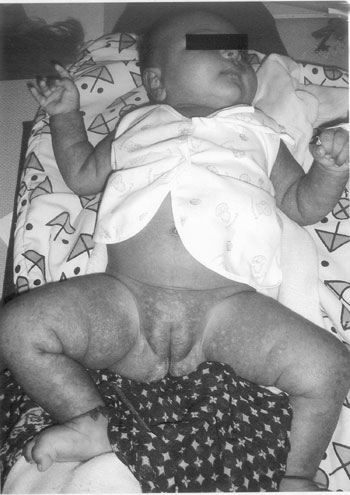 |
|
Fig. 1 Hemorrhagic rash of rickettsial
infection.
|
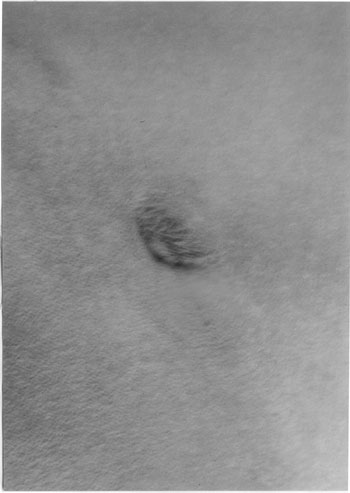 |
|
Fig. 2 Eschar in left inguinal region.
|
Eschar: A necrotic
eschar at the inoculating site is seen in variable
proportion of Indian tick tuphus, scrub typhus and
rickettsialpox cases. The site of initial tick bite
is inapparent in other rickettsial infections.
Eschar, a black necrotic area, resembles the skin
burn of cigarette butt (Fig.2). A
necrotic eschar usually has an erythematous rim and
is associated with regional lymphadenopathy.
Generalised lymphadenopathy and
hepato-splenomegaly are seen in majority of scrub
typhus patients(19).
Systemic features: Clinical
features referable to various systems are sometimes
seen in rickettsial infections. Gastrointestinal
symptoms including nausea, vomiting, abdominal pain
and diarrhea are seen with varying frequency.
Constipation is seen particularly in epidemic
typhus. Respiratory symptoms include cough and
distress are sometimes seen. Neurological
manifestations like dizziness, drowsiness,
disorientation, tinnitus, photophobia, delirium,
meningismus, and visual disturbances; are seen more
commonly with typhus group rickettsioses. The word
‘typhus’ refers to cloudy state of consciousness (‘typhos’:
cloud or smoke).
Miscellaneous: Periorbital
edema, conjunctival hyperemia, epistaxis, acute
reversible hearing loss and arthralgia are sometimes
reported.
Severe Manifestations and
Complications
Rickettsial infections sometimes
produce severe life threatening manifestations and
takes a fulminant course. Fulminant course of
rickettsial infections, particularly spotted fever
group is known to occur in patients with
glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Following are the life threatening manifestations of
rickettsial infections.
1. Respiratory:
Interstitial pneumonitis and noncardiogenic
pulmonary edema secondary to pulmonary microvascular
leakage are occasionally observed.
2. Neurological:
Meningoencephalitic syndrome is known to occur with
rickettsial infections. In fact, rickettsial
infections should be included in differential
diagnosis of aseptic meningitis and encephalitis in
patients exposed to endemic areas specially when
accompanied by renal insufficiency and/or
jaundice(20, 21).
3. Renal: Acute renal
failure is associated with bad prognosis and can be
a presenting feature of rickettsial disease. The
possibility of scrub typhus should be borne in mind
whenever a patient of fever present with varying
degree of renal insufficiency particularly if eschar
exists alongwith history of environmental
exposure(22,23).
4. Disseminated intravascular
coagulation like syndrome, hepatic failure,
gangrene and myocarditis are sometimes seen in
rickettsioses.
Laboratory Findings
No single laboratory finding is
specific for early diagnosis. Various laboratory
abnormalities found in rickettsial diseases are
described below.
Hematology: Total leucocyte
count, during early course of the disease, is normal
to low normal with marked shift to left. Later in
the course of the disease, it shows leucocytosis in
30% of cases(24). Low platelet counts are present in
about 60% cases(16). Erythrocyte sedimentation rate
is usually high.
Biochemistry: Hyponatremia
and hypoalbumi-nemia, reflecting increased vascular
permiability, are sometimes helpful in
differentiating rickettsial infections from other
acute infections. Thrombo-cytopenia, hyponatremia
and normal to low leuco-cyte count are certain clues
to early diagnosis. Hepatic transaminase values are
frequently elevated. Blood urea is elevated due to
prerenal mechanisms.
Serology:
Microimmunoflorescence, immuno-peroxidase assay,
latex agglutination, indirect hemagglutination,
enzyme-linked immunosorbent assay, dot blot
immunoassay (including dipstick test) and Weil-Felix
test are the various serological methods available
for diagnosis of rickettsial diseases. Of these,
only Weil-Felix test is easily available in India.
As all these tests detect antibodies, they would be
able to make diagnosis only after 5-7 days of onset
of disease and hence play no role for initiation of
therapy in a suspected case.
(a) Weil-Felix test:
The sharing of antigens between rickettsia and
proteus is the basis of this heterophile antibody
test. It demonstrates agglutinins to Proteus
vulgaris strain OX 19, OX 2 and OX K. Most of
the Western literature has advised against
performing this test for diagnosis of rickettsial
infections(12). The poor sensitivity of the WF
test is now well demonstrated but a good
correlation between the results of the WF test and
detection of IgM antibodies by an indirect
immunofluorescence assay (IFA) is often
observed(25). This can be used as a screening
test, which detects more cases than misdiagnosed
ones and when positive, is reasonably specific. In
spite of all its drawbacks, Weil-Felix test still
serves as a useful and cheap diagnostic tool for
laboratory diagnosis of rickettsial disease(1).
Either four fold rise in agglutinin titre in
paired sera or single titre of more than 1:320 is
considered diagnostic for infection with these
febrile agents. The use of this test is accepted
in conditions where definitive investigations are
not available(26,27). Isaac, et al.(28)
have demonstrated that the sensitivity of
Weil-Felix was 30% at a breakpoint titre of 1:80,
but the specificity and positive predictive value
were 100%. Hence Weil-Felix test is still not
entirely obsolete but has to be interpreted in the
correct clinical context(6).
(b) IFA: This is a
reference serological method for diagnosis of
rickettsial diseases and is considered ‘gold
standard’. It is not available in India. As with
all other serological methods, it usually provides
retrospective diagnosis and sensitivity is
enhanced by testing paired sera (acute and
convalescent).
Polymerase chain reaction assay:
It can be used to detect rickettsial DNA in whole
blood, buffy coat fraction or tissue specimen. It is
the most rapid assay for the diagnosis. It has
certain disadvantages like varying levels of
sensitivity, high cost and nonavailability.
Immunohistochemistry and
isolation of organism: in cell culture or
laboratory animals are other methods restricted to
research laboratories.
Diagnosis
No rapid laboratory tests are
available to diagnose rickettsial infection early in
the course of disease. It is emphasized again that
the only crucial factor for early diagnosis is high
index of suspicion. Following five factors taken
together should help in diagnosis, which can then be
confirmed with serology.
1. Compatible clinical
presentations: Various clinical situations where
a diagnosis of rickettsial disease should be
considered are fever without source, pyrexia of
unknown origin (PUO), fever with rash (rash which is
petechial, involving palms and soles, having
centripetal spread), fever with eschar,
meningoencephalitis or aseptic meningitis, acute
renal insufficiency with eschar, and infective
vasculitidis.
2. Tick bite or tick
exposure: Tick bite is painless and histoty of
tick bite is present in less than 50% of cases.
Hence absence of tick bite should not dissuade a
pediatrician from considering the diagnosis of
rickettsial disease. Patient should be completely
exposed to look for ticks on body and clothing.
Outdoor actvities in areas with high uncut grass,
weeds, low bushes or animal sheds where ticks are
often seen is a definite risk factor. Contact with
family dog in whom history of tick attachment or
tick removal is forthcoming can be useful.
3. Epidemiological data:
Diagnosis should be considered in areas known for
rickettsial disease. But in absence of multicentric
studies, one would not know prevalence in particular
area. Occurrence of similar illness (like index
case) simultaneously or sequentially in family
members or family pets can be a useful link as small
‘islands’ of infected ticks may occur in discrete
geographic units such as neighborhood or parks(16).
4. Suggestive laboratory
features: Normal to low leucocyte count with
marked left shift, thrombocytopenia, hyponatremia
and mildly elevated hepatic transaminases are
compatible with diagnosis of rickettsial disease,
although absence of these does not rule it out.
5. Rapid defervescence with
appropriate antibiotics: It is so characteristic
that it can be used as a diagnostic test for
rickettsial disease. In fact if fever fails to
respond in 48 hours, one should review the
diagnosis. Severely ill patients with multiple organ
dysfunction may take longer period of time to
respond.
Thus, rickettsial infection
should be suspected in presence of above clinical
features in a patient with likelihood of tick
exposure. They should undergo relevant hematological
and biochemical testing and those with high
probability of rickettsial infection should be
treated with appropriate antimicrobials. Failure of
defervescence within 48 hours should lead to search
for alternative diagnosis.
Differential Diagnosis
Rickettsial diseases can be
easily confused with a variety of viral (measles,
enteroviral exanthems, dengue, infectious
mononucleosis), protozoal (malaria), bacterial
(meningococcemia, typhoid, leptospirosis, toxic
shock syndrome, scarlet fever) and collagen vascular
(Kawasaki disease, other vasculitis) diseases, and
adverse drug reactions. Invasive meningococcal
disease may not be reliably distiguished from
rickettsial disease clinically, hence one may need
to treat for both conditions, after sending
cerebrospinal fluid and blood for appropriate
studies(4). The possibility of rickettsial disease
should be considered in those leptospirosis patients
who present with atypical features or respond poorly
to therapy(29).
Treatment
Definitive treatment should be
instituted on the basis of clinical and
epidemiological clues as early as possible to avoid
severe disease and fatal outcome(30,31). Various
antibiotics useful for treating rickettsial diseases
are tetracyclines, preferably doxycycline,
chloramphenicol, macro-lides(32,33) specially,
azithromycin, clarithromycin, roxythromycin, and
fluroquinolones, specially ciprofloxacin, ofloxacin,
pefloxacin, levofloxa-cin(12). Doxycycline is the
drug of choice. Oral treatment is used unless
patient is vomiting or obtunded. Dose is 5 mg/kg/day
in two divided doses for children below 45 kg and
200 mg/day in two divided doses for children above
45 kg. Duration of therapy should be at least 3 days
after defervescence or minimum 5-7 days.
Use of tetracycline to treat
children below 8 years is no longer a subject of
controversy (34-36). It has been observed that
cosmetically perceptible staining of teeth require
six or more multiple day courses of therapy. Because
TBRD can be life threatening and limited courses
with tetracycline class antibiotics donot pose a
substantial risk for tooth staining, the American
Academy of Pediatrics committee on infectious
diseases revised it’s recommendations in 1997 and
has identified doxycycline as the drug of choice for
treating presumed or confirmed RMSF in children of
any age(37).
Chloramphenicol has more side
effects and needs hematological monitoring. On many
occasions, fluroquinolones are associated with
clinical failures despite good in vitro activity.
Clarithromycin can be considered a valid alternative
to tetracycline and chloramphenicol, especially for
children less than 8 years of age(32,33). Occasional
cases with resistance to doxycycline are treated
with macrolides or rifampin. Sulfonamides are
contraindicated in rickettsial diseases as they
increase morbidity and mortality either by delaying
institution of appropriate antibiotics or directly
stimulating the growth of organisms.
Good supportive therapy is needed
in critically ill patients as iatrogenic cerebral
and pulmonary edema is easily precipitated due to
preexsisting micro-vascular leakage. Judicious use
of corticosteroids is advocated by some in
meningoencephalitis(12). Supportive care is also
needed for hypovolemia, coagulopathy, seizures and
intercurrent infections.
Contributors: NR: concept,
design, analysis and acquisition of data, revised
manuscript for important intellectual content, will
act as guarantor. AR: review of literature, drafting
and microbiological aspects. Final manuscript was
approved by both authors.
Funding : None.
Competing interest : None
stated.
|
Key Messages
•
Rickettsial infections are prevalent in
various parts of India.
•
These are one of the most difficult infections
to diagnose in their early course and high
index of suspicion is the key to early
diagnosis.
•
Fever, rash, headache, myalgia,
lymphadenopathy and eschar are various
clinical features of these infections.
•
Epidemiological features and history of
exposure to vector are crucial for diagnosis.
•
Failure of early diagnosis is associated with
significant mortality and morbidity and also
leads to expensive PUO workup.
•
Therapy is easy and affordable with dramatic
results and needs to be started on clinical
suspicion, as there is no specific test for
early diagnosis.
•
Doxycycline is the drug of choice and it can
be used safely even in children below 8 years
of age. |
References
1.
Batra HV. Spotted fevers and typhus fever in Tamil
Nadu – commentary. Indian J Med Res 2007; 126:
101-103.
2. Jayaram Paniker CK.
Ananthanarayan and Paniker’s Textbook of
Microbiology. 7th ed. University Press Pvt Ltd;
2008; 412-421.
3. Kelly DJ, Richards A, Temenak
J, Strickman D, Dasch GA. The past and present
threat of rickettsial disease to military medicine
and international public health. Clin Infect Dis
2002; 34 (suppl 4): S 145-169.
4. Chapman AS, Bakken JS, Folk
SM, Paddock CD, Bloch KC, Krusell A, et al.
Diagnosis and management of tickborne rickettsial
diseases. MMWR Recomm Rep 2006; 55: 1-27.
5. Chugh TD. Emerging and
reemerging bacterial diseases in India. J Biosci
2008; 33: 549-555.
6. Mahajan SK, Kashyap R, Kanga
A, Sharma V, Prasher BS, Pal LS. Relevance of
Weil-Felix test in diagnosis of scrub typhus in
India. J Assoc Phys India 2006; 54: 619-621.
7. Mathai E, Lloyd G, Cherian T,
Abraham OC, Cherian AM. Serological evidence of
continued presence of human rickettsiosis in
southern India. Ann Trop Med Parasitol 2001; 95:
395-398.
8. Sundhindra BK, Vijaykumar S,
Kutti AK. Rickettsial spotted fevers in Kerala. Natl
Med J India 2004; 17: 51-52.
9. Padbidri VS, Rodrigues JJ,
Shetty PS. Tick-borne rickettsiosis in Pune
district, Maharashtra, India. Int J Zoonoses 1984;
11: 45-52.
10. Centre of Disease Control and
Prevention (CDC). Rickettsial Diseases. Available at
http:// www.cdc.gov/ncidod/diseases/sunmenus/sub_rickettsial.htm.
Accessed 30 May, 2009.
11. Gregory AD, Jennifer HM.
Other rickettsia species. In: Sarah SL, Larry
KP, Charles GP, editors. Principles and Practice of
Pediatric Infectious Diseases. 2nd ed. Philadelphia:
Churchill Livingstone; 2003. p. 945-951.
12. Siberry GK, Dumler JS.
Rickettsial infections. In: Kliegman
RM, Behrman RE, Jenson HB, Stanton BF, editors.
Nelson Textbook of Pediatrics, 18th
ed. Pennsylvania, Saunders; 2007. p. 1289-1301.
13. Walker DH. Rickettsiae and
rickettsial infections : current state of knowledge.
Clin Infect Dis 2007; 45 Suppl 1: S39-44.
14. Sreeja P, Elizabeth M,
Prabhakar DM. Scrub typhus. Indian Pediatr 2004; 41:
1254-1257.
15. Somashekar HR, Prabhakar DM,
Sreeja P, Elizabeth M, Didier R, Jean MR. Magnitude
and features of scrub typhus and spotted fever in
children in India. J Trop Pediatr 2006; 52: 229.
16. Christopher DP, James EC.
Rickettsia rickettsii. In: Sarah SL, Larry KP,
Charles GP, editors. Principles and Practice of
Pediatric Infectious Diseases. 2nd ed. Philadelphia:
Churchill Livingstone; 2003. p. 942-945.
17. Walker DH, Raoult D.
Rickettsia rickettsii and other spotted fever group
rickettsiae. In: Mandell GL, Bennet JE,
Doalin R, Editors. Principles and Practice of
Infectious Diseases. Philadelphia: Churchill
Livingstone; 2000. p. 2035-2042.
18. Sexton DJ, Corey GR. Rocky
Mountain "Spotless" and "almost spotless" fevers: a
wolf in sheep’s clothing. Clin Infect Dis 1992; 15:
439-448.
19. Sirisanthana V, Puthanakit T,
Sirisanthana T. Epidemiologic, clinical and
laboratory features of scrub typhus in thirty
Thai children. Pediatr Infect Dis J 2003; 22:
341-345.
20. Kim DE, Lee SH, Park KI,
Chang KH, Roh JK. Scrub typhus encephalomyelitis
with prominant focal neurological signs. Arch Neurol
2000; 57: 1770-1772.
21. Silpapojakul K, Ukkachoke C,
Krisanapan S, Silpapojakul K. Rickettsial meningitis
and encephalitis. Arch Intern Med 1991; 151: 1753.
22. Tsay RW, Chang FY. Serious
complications of scrub typhus. J Microbiol Immunol
Infect 1998; 31: 240-244.
23. Yen TH, Chang CT, Lin JL,
Jiang JR, Lee KF. Scrub typhus: a frequently
overlooked cause of acute renal failure. Ren Fail
2003; 25: 397-410.
24. Kaplowitz LG, Fischer JJ,
Sparling PF. Rocky Mountain spotted fever: a
clinical dilemma. Curr Clin Top Infect Dis 1981; 2:
89-108.
25. La Scola B, Raoult D.
Laboratory diagnosis of rickettsioses: Current
approaches to diagnosis of old and new rickettsial
diseases. J Clin Microbiol 1997; 35:
2715–2727.
26. Fernandez AD, Johan-lian R.
Scrub typhus. E-medicine J, 2002; [email protected].
27. Suzuki T, Eto M. The value of
Weil-Felix test in the diagnosis of tsutsugamushi
disease. Jpn Med News 1980; 2956: 43-47.
28. Isaac R, Varghese GM, Mathai
E, Manjula J, Joseph I. Scrub typhus: prevalence and
diagnostic issues in rural Southern India. Clin
Infect Dis 2004; 39:1395-1396.
29. Watt G, Jongsakul K,
Suttinont C. Possible scrub typhus coinfection in
Thai agriculture worker hospitalised with
leptospirosis. Am J Trop Med Hyg 2003; 68: 89-91.
30. CDC. Consequences of delayed
diagnosis of RMSF in children. MMWR 2000; 49:
885-888.
31. Kirkland KB, Wilkinson WE,
Sexton DJ. Therapeutic delay and mortality in cases
of RMSF. Clin Infect Dis 1995; 20: 1118-1121.
32. Claudid C, Laura S, Valentino
FP, Raffaella R, Lucio T. Mediterranean spotted
fever: clinical and laboratory characteristics of
415 Sicilian children. BMC Infect Dis 2006; 6: 60.
33. Cascio A, Colomba C, Di Rosa
D, Salsa L, Di Martino L, Titone L. Efficacy and
safety of clarithromycin as treatment for
Mediterranean spotted fever in children : a
randomised controlled trial. Clin Infect Dis 2001;
33: 409-411.
34. Lochary ME, Lockhart PB,
Williams WT JR. Doxycycline and staining of
permanent teeth. Pediatr Infect Dis J 1998; 17:
429-431.
35. Grossman ER, Walchek A,
Freedman H. Tetracycline and permanent teeth: the
relation between dose and tooth colour. Pediatrics
1971; 47: 567-570.
36. Abramson JS, Ginver LB.
Should tetracyclines be contraindicated for
treatment of presumed RMSF in children less than 9
years of age. Pediatrics 1990; 86: 123-124.
37. AAP Committee on Infectious Diseases. Rocky
Mountain Spotted Fever. In: Red Book. 27th
Ed. Elk Grove Village, IL: AAP; 2006. p. 570-572.

