|
|
|
Indian Pediatr 2010;47: 145-147 |
 |
Validation of CRIB II for Prediction of
Mortality in Premature Babies |
|
Pallav Kumar Rastogi, V Sreenivas* and Nirmal Kumar
From St Stephen’s Hospital, Department of Neonatology and
Pediatrics, Tis Hazari, Delhi, India; and
*Department of Biostatistics, All India Institute of Medical Sciences,
Ansari Nagar, Delhi , India.
Correspondence to: Dr Nirmal Kumar, 4, Rajpur Road, Qtr
No B-2, Tis Hazari, Delhi 110 054, India.
Email: [email protected]
Received: May 28, 2008;
Initial review: July 8, 2008;
Accepted: February 19, 2009.
Published online: 2009 May 20.
PII:S097475590800335-1
|
|
Abstract
Objective: Validation of Clinical Risk Index for
Babies (CRIB II) score in predicting the neonatal mortality in preterm
neonates £32 weeks gestational age.
Design: Prospective cohort study.
Setting: Tertiary care neonatal unit.
Subjects: 86 consecutively born preterm neonates
with gestational age £32 weeks.
Methods: The five variables related to CRIB II
were recorded within the first hour of admission for data analysis. The
receiver operating characteristics (ROC) curve was used to check the
accuracy of the mortality prediction. H-L Goodness of fit test was used
to see the discrepancy between observed and expected outcomes.
Results: A total of 86 neonates (males 59.6%;
mean birthweight: 1228±398 grams; mean gestational age: 28.3 ± 2.4
weeks) were enrolled in the study, of which 17 (19.8%) left hospital
against medical advice (LAMA) before reaching the study end point. Among
69 neonates completing the study, 24 (34.8%) had adverse outcome during
hospital stay and 45 (65.2%) had favorable outcome. CRIB II correctly
predicted adverse outcome in 90.3% (Hosmer–Lemeshow goodness-of-fit test
P=0.6). Area under curve (AUC) for CRIB II was 0.9032. In
intention to treat analysis with LAMA cases included as survivors, the
mortality prediction was 87%. If these were included as having died then
mortality prediction was 83.1%.
Conclusion: The CRIB II score was found to be a
good predictive instrument for mortality in preterm infants
£32weeks gestation.
Key Words: CRIB II, India, Neonatal Mortality, Preterm.
|
|
A variety
of risk adjustment scores have been derived and advocated for use in
assessing neonatal mortality(1). Clinical use index for babies (CRIB)
score was created to predict mortality for infants born at less than 32
weeks gestation at birth and based upon 6 variables for predicting
mortality(2). CRIB with contemporary data has been questioned because it
needs data up to 12 hours after admission thus introducing a factor of
early treatment bias. It also utilizes FiO2 which is not a true
physiological measure because it is determined by the care team. CRIB II,
an improved version of CRIB, was published recently. The new score is
meant to improve predictions for smaller, very premature infants and to
exclude variables that could be influenced by care given to the
infants(3).
We conducted this study to validate the efficacy of
CRIB II in predicting pre-discharge neonatal mortality in preterm neonates
needing intensive care.
Methods
The prospective cohort study was conducted at a
tertiary care center between October 2005 and June 2006. Study protocol
was approved by hospital ethical committee and written informed consent
was taken from parents before enrolment in the study. All preterm newborns
£32
weeks of gestation, born in the hospital and admitted to the NICU were
eligible for inclusion and were enrolled. Exclusion criteria were
gestation <23 weeks, birth weight <500 grams, lethal congenital
malformations, delivery room deaths and admission after 12 hours of birth.
Gestational age was calculated from the first day of
last menstrual period (LMP). In cases where LMP was not known, obstetric
ultrasonography was used to assess the gestational age. In cases where
both of the above were missing a gestational age assessment was made by
using the expanded new Ballard score(4). Birthweight was recorded for each
baby as soon as they arrived in the nursery or NICU for admission. This
was done using an electronic scale having a sensitivity of 10 grams.
Arterial blood gas analysis was done in all preterm babies at admission
and then as dictated by the clinical condition of the baby. Temperature
was recorded using a digital thermometer. All these parameters along with
the sex of the baby were assigned scores according to the CRIB II. The
final CRIB II score was obtained by the arithmetic sum of the individual
scores assigned. The primary outcome measured was in-hospital mortality.
Predicted mortality was compared with observed mortality.
Logistic model was used to analyze the prediction of
mortality by the CRIB II score at admission. Discrimination – that is, the
ability of the score to correctly predict survival or death – was assessed
by calculating receiver operating characteristic curves and their
associated area under the curve (AUC). An AUC value of 0.5 indicates no
ability to discriminate and larger values indicate increasing ability. A
value of 0.8 is considered good(5).
Babies discharged against medical advice (LAMA) were
also taken into account. Data were analyzed in three ways (i) cases
with known outcomes included in the analysis and excluding those to left
against medical advice (LAMA); (ii) a comprehensive analysis of all
neonates including those who left (LAMA) and assuming all those who left
would have died if they stayed back; and (iii) after including
neonates who left and assuming all those who left would have survived if
they had stayed back.
Separate ROC curves were generated for all the three
scenarios and analyzed. The Hosmer-Lemeshow Chi-square test was performed
to look for any statistically significant difference between predicted and
observed mortality. STATA 9.1 was used for data analysis.
Results
There were 88 infants admitted to NICU at or below 32
weeks gestational age during the study period. Two babies were excluded,
one because of congenital heart disease and the other because of mistaken
dates. Thus, 86 neonates (males: 51(59.3%), birthweight: 1228 ± 398 g,
gestation: 28.3± 2.4 weeks) were enrolled, of which 17 (19.8%) left
hospital against medical advise (LAMA). Among 69 neonates completing the
study, 24 (34.8%) died and 45 (65.2%) had a favourable outcome. The mean
CRIB II score was 8.29 ± 4.35 (median 8, inter quartile range 5-12).
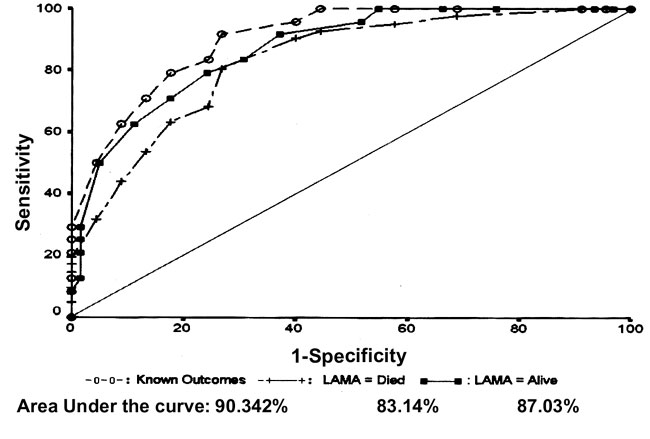
ROC curve analysis shows the area under curve (AUC) was
0.9032 (SE 0.0345, 95% CI: 0.83553-0.97096) suggesting that mortality
prediction was 90% accurate for 69 babies who stayed up to the study end
point (Fig.1). When the analysis was done assuming all those
who left were survivors up to discharge, the AUC was 0.8703 (SE 0.0394,
95% CI: 0.7931- 0.9474) suggesting mortality prediction was correct in
87%. In the analysis which included LAMA cases as died, the area under ROC
curve was 0.8314 (SE 0.043, 95% CI: 0.7468 – 0.9158) suggesting mortality
prediction was 83.1% correct.
 |
|
Fig.1
Mortality prediction on ROC
curve for different outcomes. |
HL goodness of fit test was applied to test the
difference between observed and expected outcome. There was no significant
difference between expected and observed outcome (P=0.62).
Discussion
In our study, the area under ROC curve for mortality
prediction by CRIB-II was 0.9 and there was no significant difference
between predicted and observed mortality. This is similar to the study by
Gagliardi, et al.(6), who showed AUC of 0.907. In our study
mortality prediction was better than the development study for CRIB II(3),
probable reason for this difference was related to higher mortality (33%
vs 9%) and small sample size in our study.
CRIB has previously been evaluated at our center, the
area under ROC curve was 0.823(7). The CRIB II has performed better than
CRIB in our center. A study by Christoph, et al.(8) showed
prediction with CRIB II (AUC of 0.69) was less than CRIB (0.82),
birthweight (0.74) and gestational age (0.71). The reason for the low
prediction of CRIB II in their study is not clear.
None of the babies in our study received surfactant
immediately after birth. The fact that the prediction of
survival/mortality was excellent using CRIB II suggests that survival
depends primarily on the condition of the baby at birth rather than the
intervention used. This validates the primary premise of the workers who
have developed this severity of illness score. Although CRIB II score is
less affected by perinatal factors(6) and despite good mortality
prediction, we need further studies to document the influence of various
pre and perinatal factors. A study having controlled for variables like
antenatal steroids, maternal illness, multiple pregnancy, APGAR score at
birth and use of surfactant is needed.
Contributors: PKR: design, data collection and
interpretation, manuscript writing; VS: data analysis and interpretation;
NK: concept, design, manuscript drafting and will act as guarantor of
study.
Funding: None.
Competing interests: None stated.
References
1. Ridley SA. Uncertainty and scoring system.
Anaesthesia 2002; 57: 761-767.
2. International Neonatal Network. The CRIB (Clinical
Risk Index for Babies) Score. A tool for assessing initial neonatal risk
and comparing performance of neonatal intensive care units. Lancet 1993;
342: 193-198.
3. Parry G, Tucker J, Tarnow Mordi W. CRIB II: An
update of the clinical risk index for babies score. Lancet 2003; 361:
1789-1791.
4. Ballard JL, Khoury JC, Wedig K, Wang L,
Eilers-Walsman BL, Lipp R. New Ballard’s score expanded to include
extremely premature infants. J Pediatr 1991; 119: 417-423.
5. Hanley JA, Mcneil BJ. The meaning and the use of the
area under a receiver operating characteristic (ROC) curve. Radiology
1982; 143: 29-36.
6. Gagliardi L, Cavazza A, Brunelli A, Battagliolo M,
Merazzi D, Tandoi F, et al. Assessing mortality risk in very low
birth weight infants: a comparison of CRIB, CRIB II, and SNAPPE II. Arch
Dis Child Fetal Neonatal Ed 2004; 89: F 419-422.
7. Khanna R, Taneja V, Singh SK, Kumar N, Sreenivas V,
Puliyel JM. The clinical risk index of babies (CRIB) score in India.
Indian J Pediatr 2002; 69: 657-660.
8. Christoph B, Boris M, Michael O. CRIB, CRIB II,
birthright or gestational age to assess mortality risk in very low birth
weight infants? Acta Paediatr 2008; 97: 899-903.
|
|
|
 |
|

