|
|
|
Indian Pediatr 2010;47: 131-137 |
 |
Light-emitting Diodes versus Compact
Fluorescent Tubes for Phototherapy in Neonatal Jaundice: A
Multi-center Randomized Controlled Trial |
|
Praveen Kumar, Srinivas Murki*, GK Malik†, Deepak
Chawla$, Ashok K Deorari**, N
Karthi, Sreeram Subramanian**, Jonnala Sravanthi*,
Pramod Gaddam* and SN Singh†
From the Departments of Pediatrics; Post Graduate
Institute of Medical Education and Research, Chandigarh; *Fernandez
Hospital, Hyderabad, †Chattrapati Shahuji Maharaj Medical University ,
Lucknow; $Government Medical College, Chandigarh; and **All India
Institute of Medical Sciences, New Delhi, India.
Correspondence to : Prof Ashok K Deorari, Department of
Pediatrics, All India Institute of Medical Sciences,
New Delhi, India.
Email:
[email protected]
Received: September 19, 2008;
Initial review: October 18, 2008;
Accepted: January 7, 2009.
Published online: 2009 May
20.
CTRI No. CTRI/2008/091/000072
PII:S097475590800565-1
|
|
Abstract
Objective: To evaluate whether light-emitting
diode (LED) phototherapy is as efficacious as compact fluorescent tube (CFT)
phototherapy for the treatment of non-hemolytic jaundice in healthy term
and late preterm neonates.
Study design: Multi-centre open-label randomized
controlled trial.
Setting: Four tertiary care neonatal units.
Subjects: Healthy term and late preterm neonates with non-hemolytic
jaundice.
Intervention: Single-surface LED or CFT
phototherapy.
Primary outcome variable: Duration of
phototherapy.
Results: A total of 272 neonates were randomized
to receive LED (n=142) or CFT (n=130) phototherapy. The
baseline demographic and biochemical variables were similar in the two
groups. The median duration of phototherapy (IQR) in the two groups was
comparable (26 (22-36) h vs. 25(22-36) h; P=0.44). At any time
point, a similar proportion of neonates were under phototherapy in the
two groups (log-rank test, P=0.38). The rate of fall of serum
total bilirubin (STB) during phototherapy and the incidence of ‘failure
of phototherapy’ were also not different. An equal proportion of
neonates had a rebound increase in STB needing restarting of
phototherapy. Side effects were rare, comparable in the two groups and
included hypothermia, hyperthermia, rash, skin darkening and
dehydration.
Conclusions: LED and CFT phototherapy units were
equally efficacious in the management of non-hemolytic
hyperbilirubinemia in healthy term and late-preterm neonates.
Key Words: Compact fluorescent tube, Jaundice, Light emitting
diode, Neonate, Phototherapy.
|
|
Among hospital born neonates in India, 3%
develop serum total bilirubin (STB) levels more than 15 mg/dL(1).
Phototherapy is the main treatment for neonatal hyperbilirubinemia. It is
most effective in lowering serum bilirubin when wavelength of the light
output is in blue to green spectrum (420 to 490 nm)(2). However, there is
no standard method of delivering phototherapy. The efficacy of
photo-therapy depends on light-source characteristics like emission peak
wave-length, emission range and irradiance, apart from various clinical
factors(3). The conventional phototherapy units have limited capacity to
produce high irradiance and also generate considerable heat. Gallium
nitride derived light emitting diodes (LED) which emit high intensity
light of narrow wavelength spectrum and produce minimal heat have recently
been utilized as light sources in phototherapy units. These units can be
placed very close to the neonate without any untoward effects. They are
also durable light sources with an average life of 20,000 hours. These
unique characteristics of LEDs make them an attractive light source for
the optimal phototherapy unit. Although LED devices have been shown to be
effective in in vitro studies, the clinical data comparing LEDs
with conventional units is limited(4-9). Hence, we conducted this trial to
answer the question "whether LED phototherapy is as efficacious as the
standard compact fluorescent tube (CFT) phototherapy in management of
healthy term and late preterm neonates with non-hemolytic jaundice".
Methods
This was an open-label multi-center randomized
controlled trial conducted in four tertiary care neonatal units across
India, from November 2007 to July 2008. The study protocol was approved by
institutional ethics committees of all the four hospitals and the study
was registered with Clinical Trial Registry of India. A written informed
consent was obtained from one of the parents before enrolment.
Subjects: Newborn infants born at 35 or more
completed weeks of gestation were eligible for enrolment, if they
developed hyperbilirubinemia needing phototherapy within first 7 days of
life. The decision to start phototherapy was made by bedside physicians on
the basis of the age of the baby in hours and STB levels, as per American
Academy of Pediatrics guidelines(3). Phototherapy was stopped when two
consecutive STB levels, measured 6 hours apart were less than 15 mg/dL.
Infants with perinatal asphyxia (Apgar score <4 at 1 minute or <7 at 5
minute), onset of jaundice within 24 h of age, evidence of hemolysis
(positive direct Coombs test), rhesus hemolytic disease, culture-positive
or clinical sepsis, need for exchange transfusion at the time of
enrolment, and major congenital malformations were excluded.
Intervention: Enrolled infants were randomized to
receive single surface LED or CFT phototherapy. A web-based random number
generator was used for block randomization stratified for each center(10).
The site investigator allocated the group by opening serially numbered,
opaque, sealed, identical envelopes containing the treatment group
allocation after obtaining the informed consent. The prototype LED
phototherapy units (Srichakra Scientifics, Hyderabad) had multiple LED
bulbs arranged in an area of about 20×15 cm. The units were pre-tested by
Electronics Regional Test Laboratory (East), Government of India at
Kolkata and showed peak emission wavelength between 461 to 467 nm.
Commercially available CFT units consisting of 6 special blue compact
fluorescent bulbs (18W, OSRAM special blue lamp) were used for the study.
Two phototherapy units of each type were designated as ‘study machines’ at
each center and were available for the study cohort. An eligible infant
was enrolled only if at least one phototherapy unit of each type was
available at the given time. At the beginning of the enrolment, new lamps
were installed in all the units. The CFL lamps were replaced during the
study period as and when they were visibly discolored or were producing
less light or when the irradiance fell to less than 15 µW/cm 2/nm.
The LED lamps were not changed during the study period. In both the
groups, each enrolled neonate received phototherapy using a single
overhead phototherapy unit. A distance of 25-30 cm was maintained between
the baby and the bulb/lamp surface for both type of units. Site
investigators were free to provide additional therapy for
hyperbilirubinemia like fluid/feed supplementation and phenobarbitone. In
all the centers, the study babies were cared for in wards with
environmental temperature control. Radiant heaters or blowers were used as
and when required.
Outcome variables: The duration of phototherapy was
the primary outcome. It was calculated by subtracting age at start of
phototherapy from age at end of phototherapy in hours. Brief periods of
discontinuation of phototherapy for feeding the baby or changing nappy
were not excluded while calculating total duration of phototherapy. The
secondary outcomes were failure of phototherapy, rate of fall of STB and
occurrence of hypothermia. ‘Failure of phototherapy’ was defined as STB
rising or becoming more than 20 mg/dL during phototherapy, which required
either use of double surface phototherapy or exchange transfusion. ‘Rate
of fall of STB’ was calculated by dividing the difference between STB at
start and end of phototherapy with duration of phototherapy.
Data collection and monitoring: Clinical monitoring
was done for side effects of phototherapy like dehydration and skin rash;
and a 4-hourly axillary temperature measurement was done to detect
episodes of hypothermia or hyperthermia. STB was measured every 6 to 8 h
using bilirubinometers based on direct spectrophotometery (Twin-beam
micro-bilimeter, Ginevri Technologie Biomediche; Italy or Unibeam
microbilimeter, Ginevri Technologie Biomediche, Italy; or Bil-100, Cosmo
Medical, Korea; coefficient of variation 1 to 3%, range of bilirubin
measurement 0-30 to 0-40 mg/dL). Apart from daily internal calibration,
the bilirubinometers were checked 3-monthly against low and high bilirubin
standards supplied by the respective manufacturers. However,
bilirubinometers used at different centers were not compared against each
other on the same sample in the same laboratory. In one center (PGIMER,Chandigarh),
the irradiance of the phototherapy units at the surface of the babies was
checked at the level of face, xiphoid and knees by a photoradiometer (Fluoro-lite
451, Minolta/Air Shields, USA) at the initiation of phototherapy, and then
once a day for all babies. In the other 3 centers, only periodic checks of
irradiance were done for monitoring and their data was not included for
calculating the spectral irradiance.
Sample size: In a previous unpublished trial using
CFT phototherapy units in non-hemolytic jaundice, the mean duration of
phototherapy was 25.3±14 h. To prove, with 80% power and alpha of 0.05,
that duration of phototherapy with LED phototherapy unit is not different
by more than 6h, we needed to enroll 125 subjects in each group (Power and
Precision software ver 2.0, Biostat Inc., USA).
Statistical analysis: Data entry and analysis were
done using Epi Info (CDC, Atlanta). Continuous data with normal
distribution was analyzed by student t-test and non-normally
distributed data by Mann Whitney U test. Categorical data was analyzed by
chi-square or Fisher exact test. A P value of <0.05 was
taken as significant. Analysis was intention-to-treat. It was decided a
priori that LED will be considered equally efficacious if the
difference in duration of phototherapy between the two groups is less than
6 hours without any increase in adverse effects.
Results
The study enrollment is depicted in Fig.1.
The mean birthweight and gestation of enrolled and excluded neonates were
comparable. There were 39, 37, 35 and 31 neonates in LED and 39, 35, 34
and 22 neonates in CFT groups at AIIMS New Delhi, FH Hyderabad, PGIMER
Chandigarh and CSMMU Lucknow, respectively. There were 9 exceptions to the
protocol. Three neonates were enrolled despite low Apgar score (but had no
hypoxic ischemic encephalopathy) and six despite the presence of sepsis.
The results with and without these cases were comparable and analysis
reported here includes these 9 subjects.
The birthweight, gestation and other demographic
variables were similar in the neonates enrolled in LED or CFT groups (Table
I). The infant characteristics and laboratory parameters at the start
and during phototherapy, which may have impact on the duration of
phototherapy were also comparable in the two groups, except for spectral
irradiance which was higher in LED group as compared to CFT group (Table
II). One neonate in LED group received phenobarbitone while 1 in CFT
group received extra intravenous fluids. The STB at the time of stopping
phototherapy was similar in the two groups thereby indicating that uniform
guidelines were followed.
Table I
Description of Study Population
|
Parameters |
LED ( n=142) |
CFT (n=130) |
P |
|
Mean(SD) |
n=115 |
n=110 |
|
|
Birth weight (g) |
2807(458) |
2771(489) |
0.57 |
|
Gestation (wk) |
37.6(1.4) |
37.6(1.4) |
0.88 |
|
Median (IQR) |
n=128 |
n=120 |
|
|
Apgar at 1 min |
8(7-9) |
8( 8-9) |
0.42 |
|
Apgar at 5 min |
9(9-9) |
9(9-9) |
0.60 |
|
Frequency (%) |
|
Male sex |
77 (54) |
73 (56) |
0.75 |
|
Gestational diabetes in mother |
10 (7) |
10 (8) |
0.81 |
|
Mode of delivery |
|
vaginal |
86 (61) |
80 (61) |
0.80# |
|
instrumental |
16 (11) |
11 (9) |
|
|
cesarean |
440 (28) |
39 (30) |
|
|
Setting of ABO incompatibility |
53 (37.3) |
40 (30.7) |
0.26 |
|
Rh Negative mother (No isoimmunization) |
8 (5.6) |
7 (5.4) |
0.93 |
|
Sibling with jaundice needing treatment |
3 (2.9) |
6 (6.4) |
0.31# |
|
Exclusive breastfeeding |
97 (68) |
91 (70) |
0.75 |
|
#Fischer-exact
test #Mann-Whitney test |
Table II
Baseline Variables Which May Affect Duration of Phototherapy
|
Parameters |
LED ( n=142) |
CFT (n=130) |
P |
|
Mean (SD) |
|
Weight at start of phototherapy (g) |
2644 (434) |
2591 (469) |
0.32 |
|
Age at beginning phototherapy (h) |
81.7 (35.6) |
81.4 (32.5) |
0.93 |
|
Serum bilirubin at start of phototherapy
(mg/dL) |
16.8 (2.4) |
16.9 (2.5) |
0.96 |
|
Serum bilirubin at stopping phototherapy
(mg/dL) |
12.1 (2.1) |
12.3 (1.9) |
0.65 |
|
PCV (%) at start of phototherapy |
52.2 (6) in 66 |
53.1 (6.2) in 56 |
0.43 |
|
Reticulocyte count (%) |
2.8 (4.1) in 66 |
2.0 (2.1) in 55 |
0.24 |
|
Spectral irradiance‡ |
47 (3.3) |
28.7 (2.7) |
<0.001 |
|
Median (IQR) |
|
Percent weight loss |
4.9(3.1-8.2)in 115 |
5.8(3.6-8.1) in 110 |
0.14## |
|
Frequency (%) |
|
Proportion with weight loss >10% |
13 (9.2) in 115 |
16 (12.3) in 110 |
0.40 |
|
Oxytocin use during labor |
59 (42) |
65 (50) |
0.16 |
|
Cephalhematoma |
7 (5) |
3 (2) |
0.34# |
|
G6PD deficient |
7/65 (10.8) |
3/54 (5.6) |
0.35# |
|
Top feed during phototherapy |
3 (4.6) |
4 (7.1) |
0.70# |
|
‡ Based on values obtained in 35 cases in LED and 34 cases in CFT
group; #Fischer-exact test ##Mann-Whitney test |
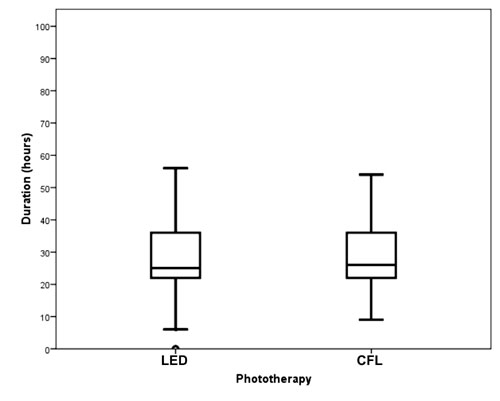
The duration of phototherapy was normally distributed
in the LED group, but was skewed positively in the CFT group (P
<0.001; D’Agostino and Balanger test for normality) due to 7 cases in this
group needing phototherapy for more than 60 hours (Fig. 2).
The median duration of phototherapy was comparable in the two groups [26
h, (IQR: 22-36) versus 25 h, (IQR: 22-36); P=0.44]. At any time
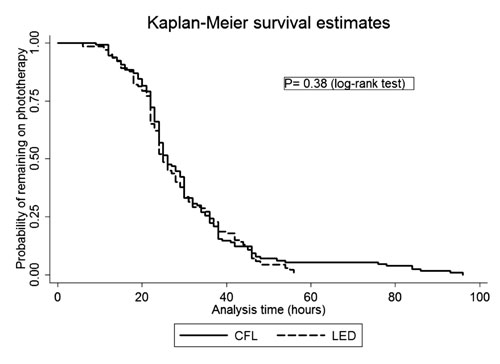
point, a similar proportion of neonates were under phototherapy in the two
groups (Fig. 3), (log-rank test, P=0.38).
 |
|
Fig. 2 Duration of phototherapy. |
|
 |
|
Fig. 3 Survival analysis: Duration of
phototherapy. |
The mean (SD) rates of fall of STB during phototherapy
were 0.19 (0.13) and 0.19 (0.14) mg/dL/h in LED and CFT groups
respectively (P=0.78). The incidence of ‘failure of phototherapy’
(6 (4.2%) vs 3 (2.3%); P=0.72) and exchange transfusion (2 (1.4%)
vs 0; P=0.50) were similar in the two groups. The subjects with
failure of phototherapy were not different from those without failure in
terms of age, weight at admission, initial PCV and initial STB. An equal
proportion of neonates had a rebound increase in STB needing phototherapy
(8 (5.6%) vs 7 (5.4%); P=0.93). Side effects were rare and
comparable in the two groups. In the LED group, 3 infants had hypothermia
(lowest temperature 36.0 şC), 4
developed hyperthermia, 1 had mild dehydration and rash was noticed in 2
infants. Six infants in CFT group developed hyperthermia while skin
darkening was seen in one neonate.
Discussion
Two hundred and seventy two healthy term and late
preterm neonates with non-hemolytic hyperbili-rubinemia were randomized to
receive LED or CFT phototherapy, across four neonatal units. The efficacy
of both types of phototherapy devices in terms of duration of
phototherapy, rate of fall of STB, incidence of ‘failure of phototherapy’
and need for exchange transfusion was similar in this trial.
There is no ‘standard’ recommended method of
administering phototherapy and a variety of strategies have been followed
by different researchers. The few earlier publications have compared
neoBLUE (Natus Inc., USA) or Super-LED (Fanem, Brazil) devices with either
halogen quartz lamps or standard BB blue tubelights(4,6-8). The studies
using a strategy of ‘similar irradiance’ for the two types of devices did
not find any difference in their efficacy(4,7,8). Martins, et al.(6)
adjusted the devices to obtain a similar exposed surface area, but a
higher irradiance in the LED group resulted in a better efficacy with LED
units. We tested an indigenously manufactured prototype LED device and
adopted a strategy of ‘similar distance’ for the two devices. Although,
the LED units had a higher spectral irradiance than CFT units, they
achieved similar efficacy. In order to explain this, we did a crude
estimation of the footprint (body surface area covered) of the two units.
The LED unit had a bulb area of 300 cm 2
while the CFT unit had a lamp area of 770 cm2. The footprint (area in
which the irradiance was >15 µW/cm2/nm) covered by the two units at
the bed level at a distance of 25 cm was 660 cm2 and 744 cm2,
respectively. Though the CFT unit covered a larger footprint, the lamp
area/surface area ratio was 0.96 as against 2.2 for LED unit. The
advantage of the higher spectral irradiance achieved with LEDs might have
been neutralized by the lesser surface area covered by these. It is
possible that if the bulb area in the LED unit is increased or the
arrangement of the bulbs is altered to increase the footprint, its
efficacy would improve further. It would be useful to investigate the
exact body surface area covered by different phototherapy units in a more
scientific manner utilizing irradiance mapping technique(11).
Since we were testing a newly introduced LED system, we
enrolled a relatively ‘low-risk’ population of healthy neonates with
non-hemolytic jaundice. Whether LED units will be similarly effective in
hemolytic jaundice is not known. The efficacy of a phototherapy system is
influenced by initial STB levels, body surface area exposed and spectral
irradiance(3). We kept LED light source at a relatively large distance
from the body surface to match the distance achievable with CFT
photo-therapy. As LED light sources do not produce much infrared light,
they can be brought much closer to the baby with potential increase in
efficacy, without danger of hyperthermia or burns.
The side effects like hypothermia and hyperthermia were
rare and comparable in the two groups. This may be partly because the
enrolled neonates were treated in temperature controlled environments with
regular monitoring of body temperature. Since LEDs do not produce much
heat, hypothermia may be a problem when used in small and sick babies, and
in environments without temperature control. In such situations, a closer
monitoring and external heat source may be required.
In different centers, STB was measured by three
different bilirubinometer models from two manu-facturers. It was not
feasible to compare the bilirubinometers used at different centers against
each other by running the same sample. However, all centers followed
regular internal calibrations and periodic checking against known
standards. The irradiance could not be measured regularly at all centers.
However, that should not affect the results since a strategy of keeping
‘similar distance’ for the two devices was being followed rather than
‘similar irradiance’.
Although phototherapy has been used for the treatment
of neonatal hyperbilirubinemia for more than four decades, the most
efficacious method with least side effects is yet to be developed. There
is a need to conduct actual cost-effectiveness studies using different
phototherapy devices. Studies are also required to compare different types
of LED devices. For better comparison, future studies should record not
only the distance and irradiance but also the body surface area covered by
them. The effects of LEDs on conversion of bilirubin to various bilirubin
isomers also needs to be studied in vitro and in vivo.
Contributors: PK, AKD, GKM, SM and DC designed the
study. KN, SS, VS and SNS recruited the subjects and collected the data.
PK, AKD, GKM, PG and SM monitored the patient recruitment and data
collection. DC and PK analyzed data and wrote the manuscript with inputs
from AKD, GKM and SM. All authors reviewed the final manuscript and made
the decision to submit the manuscript for publication.
Financial assistance: None. The prototype LED
phototherapy units at all sites were provided by Srichakra Scientifics,
Hyderabad, free of cost. CFL unit at AIIMS, New Delhi, was provided by
Phoenix Medical Systems, Chennai, free of cost.
Conflict of interest: None stated.
|
What is Already Known?
• Light Emitting Diode devices can provide higher
irradiance as compared to conventional phototherapy.
What This Study Adds ?
• Light Emitting Diode (LED) phototherapy units
are at least as efficacious and as safe as Compact Fluorescent Tube
(CFT) phototherapy units in healthy term and late-preterm neonates
with non-hemolytic jaundice.
|
References
1. National Neonatology Forum of India. National
Neonatal Perinatal Database Network. Report 2002-2003. New Delhi: 2004.
2. Ennever JF. Blue light, green light, white light,
more light: treatment of neonatal jaundice. Clin Perinatol 1990; 17:
467-481.
3. American Academy of Pediatrics. Subcommittee on
Hyperbilrubinemia. Management of hyper-bilirubinemia in the newborn infant
35 or more weeks of gestation. Pediatrics 2004; 114: 297-316.
4. Seidman DS, Moise J, Ergaz Z , Laor A, Vreman HJ,
Stevenson DK , et al. A new blue light-emitting phototherapy
device: a prospective randomized controlled study. J Pediatr 2000; 136:
771-774.
5. Vreman HJ, Wong RJ, Stevenson DK, Route RK, Reader
SD, Fejer MM, et al. Light-emitting diodes: a novel light source
for phototherapy. Pediatr Res 1998; 44: 804-809.
6. Martins BM, de Carvalho M, Moreira ME, Lopes JM.
Efficacy of new microprocessed photo- therapy system with five high
intensity light emitting diodes (Super LED). J Pediatr (Rio J) 2007; 83:
253-258.
7. Maisels MJ, Kring EA, DeRidder J. Randomized
controlled trial of light-emitting diode photo-therapy. J Perinatol 2007;
27: 565-567.
8. Seidman DS, Moise J, Ergaz Z, Laor A, Vreman HJ,
Stevenson DK, et al. A prospective randomized controlled study of
phototherapy using blue and blue-green light-emitting devices, and
conventio-nal halogen-quartz phototherapy. J Perinatol 2003; 23: 123-127.
9. Chang YS, Hwang JH, Kwon HN, Choi CW, Ko SY, Park
WS, et al. In vitro and in vivo efficacy of new blue light emitting
diode phototherapy compared to conventional halogen quartz photo-therapy
for neonatal jaundice. J Korean Med Sci 2005; 20: 61-64.
10. Research Randomizer. Available from: URL:
http://randomizer.org/form.htm. Accessed October 24, 2007.
11. Vreman HJ, Wong RJ, Murdock JR, Stevenson DK.
Standardized bench method for evaluating the efficacy of phototherapy
devices. Acta Paediatr 2008; 97: 308-316.
|
|
|
 |
|

