|
|
|
Indian Pediatr 2020;57:1166-1171 |
 |
COVID-19 in Neonates: A
Call for Standardized Testing
|
|
Sindhu Sivanandan, 1
Deepak Chawla,2 Praveen
Kumar3 and Ashok K Deorari4
for the National Neonatology Forum, India
From Departments of Neonatology, 1Jawaharlal Institute of
Postgraduate Medical Education and Research (JIPMER), Puducherry;
2Government Medical College and Hospital, and Departments of
Pediatrics, 3PGIMER, Chandigarh; and 4All India Institute of Medical
Sciences, New Delhi; India.
Correspondence to: Dr. Praveen Kumar, Professor and Head, Division of
Neonatology Department of Pediatrics, PGIMER, Chandigarh 160012, India.
Email: [email protected]
Published Online: October 24, 2020;
PII: S097475591600254
|
The limited evidence on neonatal coronavirus disease
(COVID-19) suggests that vertical transmission of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) is rare, and most
neonates seem to acquire the infection postnatally through respiratory
droplets and contact. Testing of neonates with perinatal or postnatal
exposure to COVID-19 infection plays a vital role in the early
diagnosis, management and institution of infection prevention measures
thereby cutting off the chain of epidemic transmission. A recently
concluded online neonatal COVID-19 conference conducted by the National
Neonatology Forum (NNF) of India and a nationwide online survey pointed
to substantial variation in neonatal testing strategies. We, herein,
summarize the relevant literature about the incidence and outcomes of
neonatal COVID-19 and call for a universal and uniform testing strategy
for exposed neonates. We anticipate that the testing strategy put forth
in this article will facilitate better management and safe infection
prevention measures among all units offering neonatal care in the
country.
Keywords: Nucleic acid testing, RT-PCR, Rapid antigen test,
SARS-CoV-2.
|
|
T he severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has
affected over 41 million people globally and has caused more
than 1 million deaths [1]. Pediatric cases account for 2-8% of
diagnosed coronavirus disease (COVID-19) [2], and three-quarters
acquire the disease from an infected family member. While the
disease is generally milder in children when compared to adults,
a small proportion require hospita-lization or intensive care,
and there is an increasing recognition of a Multisystem
inflammatory syndrome related to COVID-19 illness in children
(MIS-C), a severe condition with potential long-term
consequences [3,4]. The infection rate in this vulnerable group
is increasing [5], and the reported burden is likely an
under-estimate due to a higher proportion of asymptomatic
infections, and lack of standardized testing protocols. Amongst
neonates, the risk of vertical transmission is rare and most
cases are reported to be acquired horizontally from infected
contacts [6]. However, the modes of transmission and the impact
of COVID-19 among neonates is less well characterized.
The National Neonatology Forum (NNF) of India
in collaboration with Federation of Obstetric and Gynaecological
Societies of India (FOGSI), and Indian Academy of Pediatrics
(IAP) has published evidence-based recommendations for
perinatal-neonatal COVID-19 [7]. In an online NNF COVID-19
conference held on 10 July, 2020, substantial variability in
testing strategy for SARS-CoV-2 exposed neonates between centers
was evident. Following this, NNF India conducted a
cross-sectional nationwide online survey in July-August, 2020 to
investigate this variability further. The call for participation
was made via email and social media. A total 45 hospitals
responded till 20 August, 2020, of which 25 were
COVID-designated hospitals. All hospitals tested neonates born
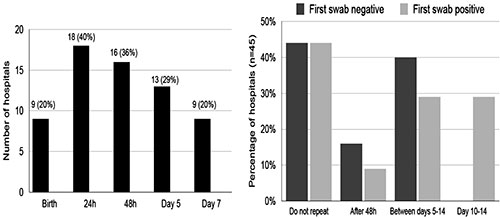
to COVID-19 positive mothers once or at multiple time points; 9
(20%) tested neonates at birth, 18 (40%) by 24 hours, 16 (36%)
by 48 hours and 49% between days 5-7 (Fig. 1).
While 44% did not do repeat testing, others repeated it after
varying time periods irrespective of initial results. The
majority (97%) used reverse-transcriptase polymerase chain
reaction (RT-PCR) test on oro-nasopharyngeal swab. Among
neonates presenting to a health facility with symptoms, 11 (25%)
of the hospitals tested all such neonates while others
selectively tested based on certain criteria like history of
contact, respiratory symptoms or as screening prior to surgery.
 |
|
(a) Timing of first RT-PCR
testing
|
(b) Timing of repeat RT-PCR
testing
|
|
Fig. 1 Testing strategies adopted by 45
participating hospitals managing neonates with perinatal
or postnatal COVID-19 exposure Results of NNF
cross-sectional online survey (July-August, 2020).
|
Lack of standardized testing protocols has
important implications for the management of the neonate,
infection prevention and control practices, as well as for
understanding disease epidemiology. In this article, we review
the available evidence on neonatal SARS-CoV-2 infection and make
a case for uniform and universal testing of COVID-19 exposed
neonates.
NEONATAL SARS-COV-2 INFECTION
Incidence
Neonates, like infants and children have a
lower incidence of SARS-CoV-2 infection. In a systematic review,
Dhir, et al. [6] reviewed the outcomes of 1141 neonates
born to COVID-19 positive women reported in 45 case series.
Two-thirds were delivered by cesarean section, a quarter were
preterm, and among 1005 (88%) neonates tested, 39 (3.9%) were
found to be positive by RT-PCR. In the perinatal COVID-19
registry of the American Academy of Pediatrics, 2962
mother-infant dyads had been enrolled till 29 August 2020 from
264 centers across the United States of America (USA) [8]. In
this registry, 2561 (86%) neonates underwent testing by RT-PCR
and 45 (1.8%) tested positive for SARS-CoV-2. Two-thirds of the
infants were delivered vaginally, and about a half were
roomed-in with their mothers, and were breastfed directly or
with expressed milk by mothers themselves. Of 26 neonatal deaths
in this cohort, none were related to COVID-19. In a large series
from Mumbai, India, only 3 out of 131 (2.2%) neonates born to
COVID-19 mothers tested positive within 24 hours of birth [9].
These neonates subsequently turned negative when retested on day
5. In this series, 50% of the infants were delivered vaginally
and rooming-in and breast-feeding were encouraged. Reports from
other national databases have shown a variable risk of perinatal
transmission; 2 (4.9%) out of 41 tested from Kuwait [10], 12
(5%) out of 240 tested from the UK Obstetric Surveillance System
(UKOSS) [11], 9 (6.1%) out of 147 tested from the Italian
Obstetric Surveillance System (ItOSS) [12], 4 (3.3%) of 120
tested from Turkey [13], and 1 (2.7%) of 36 tested (2.7%) from
France [14].
Mode of Transmission
SARS-CoV-2 infection can pass from mother to
fetus/neonate through trans-placental route or during delivery
from exposure to maternal blood or secretions. Post-natally, the
infection can be transmitted from infected mother or caregivers
through aerosols or direct contact. Initially, with limited
information, there was no con-sensus on the type of samples
(maternal and neonatal), and timing and type of testing to
categorize if the COVID-19 infection was congenital, or acquired
at birth or postnatally. Some experts have put forth
classification schema, but none is universally accepted
currently [15,16]. Generally, these are complex and mandate
serial testing to rule out surface contamination with maternal
fluids [15] or additional serological evidence of infection
[16]. Broadly, intrauterine transmission can be reasonably
confirmed, if the mother has been positive for SARS-CoV-2 within
14 days before or 2 days after birth and, the virus has been
detected in amniotic fluid, placental tissue, neonatal blood or
respiratory specimens collected within first 12 hours of birth,
as well as in repeat neonatal blood or respiratory samples after
24 hours. If the amniotic fluid, placental tissue and early
neonatal samples are negative but subsequent ones after 24 hours
are positive, it is likely that the virus was acquired
intrapartum or in early postpartum period.
Vivanti, et al. [17] made a strong
case for trans-placental transmission in a neonate who
manifested neurological symptoms on day 3 of birth. The authors
demonstrated the E and S genes of SARS-Co-V-2 in the maternal
blood and amniotic fluid along with high viral load in the
placenta and histological evidence of placental inflammation.
The nasopharyngeal and rectal swabs of the neonate collected 1
hour after birth, and then repeated on day 3 and 18 were
positive for the two SARS-CoV-2 genes [19]. The neonate improved
with symptomatic treatment and was discharged home. In another
case of a symptomatic neonate whose nasopharyngeal swab was
positive by RT-PCR at 24 and 48 hours after birth, SARS-CoV-2
nucleocapsid protein and viral particles were demonstrated in
the placental syncytiotrophoblast [18]. The neonate was
separated at birth from its COVID-19 positive mother and
subsequently recovered. Added to this conundrum is the
demonstration of SARS-CoV-19 virus-specific antibodies (IgM
and/or IgG) in the neonatal serum despite negative
nasopharyngeal swabs in a few cases [19]. While IgG antibodies
can passively transfer across the placenta, the presence of IgM
is intri-guing. Whether the elevation and rapid decline of IgM
antibody level noted in the above case represents fetal viremia
that had subsequently cleared or signaled a false-positive
result due to cross-reactivity with other viruses is a matter of
debate.
Breastmilk is unlikely to be a route of
transmission. Among 48 milk samples from 32 infected women, only
one tested positive for SARS-CoV-2 virus [20]. In two samples
produced by a single woman, IgG but not IgM antibodies against
SARS CoV 2 were detected. Cham-bers, et al. [21] showed
that mere detection of viral RNA does not equate to infectivity,
because the viral particles failed to replicate in tissue
culture [21]. Recently, secretory antibodies against SARS-CoV-2
were demons-trated in a high proportion of human milk samples
from 41 mothers with unknown COVID status during the pandemic
[22]. Possibilities include an antibody res-ponse secondary to
COVID-19 infection or the inherent characteristics of milk
antibodies to have cross-reactive and poly-reactive properties
against coronavirus and other related viruses. Thus, human milk
might have a protective role against COVID-19 illness. In the
AAP registry, the risk of COVID-19 infection among neonates
isolated at birth (22/1123; 2%) and those roomed-in (21/974;
2.2%) was similar [7]. The data from various national,
population-based studies indicate that rooming-in and direct
breastfeeding of infants born to mothers with confirmed or
suspected SARS-CoV-2 infection do not increase the risk of
infection if proper contact and droplet precautions are followed
[9,11].
Clinical Manifestations in Infected Neonates
Most neonates born to COVID-19 positive women
are asymptomatic and carry only a small risk of acquiring the
infection from mother [11,23,24]. However, they are at a higher
risk of being born preterm (30%) or by cesarean section (50% or
greater) and may require intensive care for management of
prematurity and other co-morbidities [6]. The incidence of
symptoms in neonates varies as per proportion of preterm
deliveries among different case-series and reviews. Among 58
neonates with confirmed SARS-CoV-2 infection, 22% were
asymptomatic, 41% presented with respiratory symptoms and 15%
with fever [6]. Less common symptoms included poor feeding and
lethargy (10%) and gastrointestinal symptoms (9%). The illness
manifested beyond 24 hours of age, and most improved with
symptomatic treatment. However, 38% (22 of 58 positive neonates)
required admission to neonatal unit and 17% required respiratory
support. In the AAP registry, 30% of infected neonates (n=43)
manifested COVID-19 related symptoms. The duration of
hospitalization was also longer in this group compared to
COVID-19 negative neonates. Due to the overlap of usual
morbidities of preterm and term neonates, it is difficult to
tease out the contribution of SARS-CoV-2 infection to the
reported symptoms and morbidities. Neonatal deaths due to
COVID-related illnesses are uncommon [8,11]. However, follow-up
has been reported only till hospital discharge and long-term
outcomes are not known.
Infectivity and Risk of Transmission of
COVID-19
Viral loads in children who are asymptomatic
or have mild illness have been shown to be higher than
hospitalized adults with severe disease [25]. Prolonged fecal
excretion of SARS-CoV-2 has also been shown in children and
could play a significant role in the trans-mission of COVID-19
disease [25,26]. Infected neonates may pose a higher risk to
healthcare providers and family members, especially elderly who
come in close contact with them or their excreta during
caregiving activities. Face masks are not recommended for
infants, and their care inherently requires close and repeated
contacts.
TESTING STRATEGY FOR COVID-19
Web
Table
I provides a list of diagnostic modalities for COVID-19 and
their application. The RT-PCR test to detect SARS Co-V-2 viral
genome is the preferred diagnostic modality in all age groups,
but the test should be interpreted along with clinical context.
When the pre-test probability of COVID-19 infection is high, a
single negative RT-PCR test (sensitivity, 70%; specificity, 99%)
does not help in ruling out an infection and the test needs to
be repeated. Automated RT-PCR systems (CBNAAT or TrueNat) can be
used where RTPCR testing is not available or quick turnaround is
required e.g. emergency surgery. Rapid point of care
antigen-based tests (RAT) on respiratory samples may have a role
in triaging and rapid diagnosis. However, because of low
sensitivity, if index of suspicion is high and test result is
negative, confirmatory RT-PCR testing is recommended [27]. Due
to fewer numbers of neonatal and pediatric cases, these
recommendations are extrapolated from adult data [28].
Optimal Testing Time in Neonates
We examined the data extracted from published
reports on neonates born to women with COVID-19 infection
maintained by the Cochrane Gynecology and Fertility group [29].
Similar to the findings in the NNF survey, there were variations
in the timing of testing. Therefore, the optimal testing time
proposed in this article is derived from the knowledge of the
viral infectivity and disease course in adults and children, and
the testing recommen-dations by the National COVID-19 task force
[27].
In neonates born to COVID-19 mothers, ideally
a test should be done as early as possible after birth, within
12 hours (to find out vertical transmission, only for research
purpose) and again after 5-10 days (as the initial test may have
false negatives and mother-infant dyad are generally roomed-in).
However, for those neonates who are asymptomatic and otherwise
fit to be discharged, the test can be scheduled as a
pre-discharge sample at 24-72 hours of age (to avoid delay in
discharge and missing sampling). Centers for Disease Control and
Prevention, USA has also given similar guidelines [30]. The
family should be advised to report to the nearest health
facility for a repeat test if the neonate develops any symptoms
or signs. In symptomatic neonates reporting to emergency, the
test should be done at presentation. Based on the available
evidence on neonatal SARS-CoV-2 trans-mission and the
recommendations put forth by the National Task Force on
COVID-19, we propose a testing strategy that is applicable for
India in Table I.
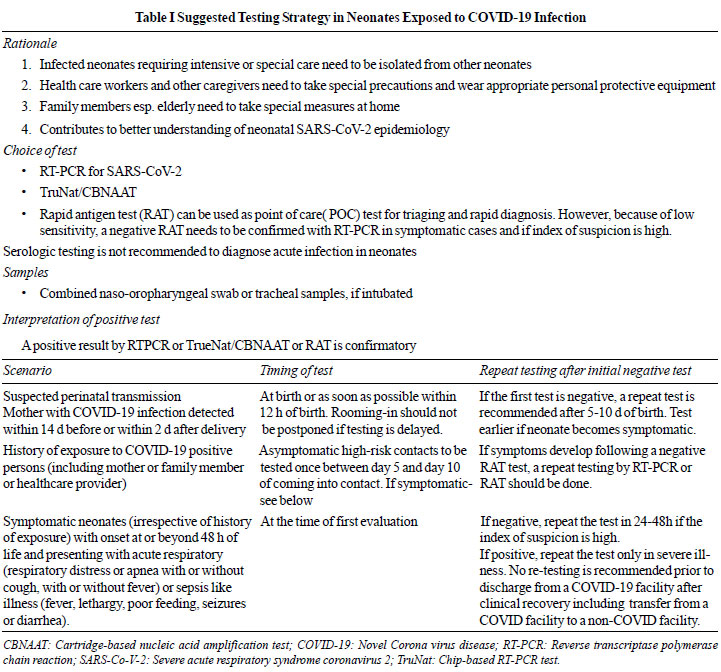 |
CONCLUSION
Although data on the incidence and outcomes
of neonatal SARS-CoV-2 infection continue to emerge, there is
much more to be learned. The evidence so far suggests that
vertical transmission is uncommon and a greater proportion
acquire infection postnatally through respiratory droplets or
contact with infected mother or care-givers. Majority of
neonates do not develop symptoms due to SARS-CoV-2 but the
morbidities related to prematurity may necessitate intensive
care and support. All neonates born to mothers with suspected or
confirmed COVID-19 infection, regardless of presence of symptoms
should be tested. The awareness about neonatal COVID status
promotes opportunities to implement infection prevention and
control measures. Cases missed through lack of clinical
suspicion or under-testing may facilitate the transmission of
SARS-CoV-2 infection because asymptomatic infected neonates may
serve as reservoirs of infection.
Contributors: All authors conceived the
idea, reviewed the manuscript, analyzed and approved the
manuscript.
Funding: None; Competing interests:
None stated.
REFERENCES
1. World Health Organization. WHO Coronavirus
Disease (COVID-19) Dashboard. Accessed 23 October 2020.
Available from: https://covid19.who.int/?gclid=EAIaIQob
ChMItPnB-82x6wIVSK6WCh35jALrEAAYASABE gKUovD_BwE.
2. Hoang A, Chorath K, Moreira A, et al.
COVID-19 in 7780 pediatric patients: A systematic review. E
Clinical Medi-cine. 2020;24:100433.
3. Jiang L, Tang K, Levin M, et al.
COVID-19 and multi-system inflammatory syndrome in children and
adole-scents. Lancet Infect Dis. 2020;20:E276-E88.
4. Gotzinger F, Santiago-Garcia B, Noguera-Julian
A, et al. COVID-19 in children and adolescents in Europe:
A multinational, multicentre cohort study. Lancet Child Adolesc
Health. 2020;4:653-61.
5. Center for Disease Control and Prevention.
Demographic Trends of COVID-19. Accessed on August 24, 2020.
Available from:
https://www.cdc.gov/covid-data-tracker/index.html#demographics
6. Dhir SK, Kumar J, Meena J, Kumar P.
Clinical features and outcome of sars-cov-2 infection in
neonates: A systematic review. J Trop Pediatr. 2020?:fmaa059
[Published online August 28, 2020].
7. Chawla D, Chirla D, Dalwai S, et al.
Perinatal-Neonatal Management of COVID-19 Infection - Guidelines
of the Federation of Obstetric and Gynaecological Societies of
India (FOGSI), National Neonatology Forum of India (NNF), and
Indian Academy of Pediatrics (IAP). Indian Pediatr.
2020;57:536-48.
8. American Academy of Pedaitrics (AAP),
Section on Neonatal Perinatal Medicine. National registry for
surveillance and epidemiology of perinatal COVID-19 infection.
Accessed 5 Sep, 2020. Available from: https://twitter.com/AAPneonatal/status/1301301988454535168/photo/1
9. Nayak AH, Kapote DS, Fonseca M, et al.
Impact of the coronavirus infection in pregnancy: A preliminary
study of 141 patients. J Obstet Gynaecol India. 2020;70:256-61.
10. Ayed A, Embaireeg A, Benawadth A, et
al. Maternal and perinatal characteristics and outcomes of
pregnancies complicated with COVID-19 in Kuwait. medRxiv
2020.07.10.20150623v1 [preprint].
11. Knight M, Bunch K, Vousden N, et al.
Characteristics and outcomes of pregnant women admitted to
hospital with confirmed SARS-CoV-2 infection in UK: National
popu-lation based cohort study. BMJ. 2020;369:m2107.
12. Maraschini A, Corsi E, Salvatore MA,
Donati S. Corona-virus and birth in Italy: results of a national
population-based cohort study. medRxiv. 2020.2006.2011.20128652
[preprint].
13. Oncel MY, Akin IM, Kanburoglu MK, et
al. A multicenter study on epidemiological and clinical
characteristics of 125 newborns born to women infected with
COVID-19 by Turkish Neonatal Society. [published online ahead of
print, 2020 Aug 10] [published correction appears in Eur J
Pediatr. 2020 Aug 22]. Eur J Pediatr. 2020;1-10.
14. Vivanti AJ, Mattern J, Vauloup-Fellous C,
et al. Retrospective description of pregnant women
infected with severe acute respiratory syndrome coronavirus 2,
France. Emerg Infect Dis. 2020;26:2069-76.
15. Shah PS, Diambomba Y, Acharya G, Morris
SK, Bitnun A. Classification system and case definition for
SARS-CoV-2 infection in pregnant women, fetuses, and neonates.
Acta Obstet Gynecol Scand. 2020;99:565-8.
16. Blumberg DA, Underwood MA, Hedriana HL,
Lakshminrusimha S. Vertical Transmission of SARS-CoV-2: What is
the Optimal Definition? Am J Perinatol. 2020;37:769-772.
17. Vivanti AJ, Vauloup-Fellous C, Prevot S,
et al. Trans-placental transmission of SARS-CoV-2
infection. Nat Commun. 2020;11:3572.
18. Sisman J, Jaleel MA, Moreno W, et al.
Intrauterine transmission of SARS-COV-2 infection in a preterm
infant. Pediatr Infect Dis J. 2020;39:e265-e7.
19. Zeng H, Xu C, Fan J, et al.
Antibodies in Infants Born to Mothers With COVID-19 Pneumonia.
JAMA. 2020. 323: 1848-9.
20. Lackey KA, Pace RM, Williams JE, et al.
SARS-CoV-2 and human milk: What is the evidence? Matern Child
Nutr. 2020:e13032.
21. Chambers C, Krogstad P, Bertrand K, et
al. Evaluation for SARS-CoV-2 in breast milk from 18
infected women. JAMA. 2020;324:1347-8
22. Demers-Mathieu V, Dung M, Mathijssen GB,
et al. Difference in levels of SARS-CoV-2 S1 and S2
subunits- and nucleocapsid protein-reactive SIgM/IgM, IgG and
SIgA/IgA antibodies in human milk. J Perinatol. 2020:1-10
[Published 2020 Sep 1].
23. Ludvigsson JF. Systematic review of
COVID-19 in children shows milder cases and a better prognosis
than adults. Acta Paediatr. 2020;109:1088-95.
24. Liguoro I, Pilotto C, Bonanni M, et
al. SARS-COV-2 infection in children and newborns: A
systematic review. Eur J Pediatr. 2020;179:1029-46.
25. Xing YH, Ni W, Wu Q, et al.
Prolonged viral shedding in feces of pediatric patients with
coronavirus disease. J Microbiol Immunol Infect. 2019;53:473-80.
26. Li X, Xu W, Dozier M, et al. The
role of children in transmission of SARS-CoV-2: A rapid review.
J Glob Health.2020;10:011101.
27. Indian Council of Medical Research
(ICMR).Advisory on Strategy for COVID-19 Testing in India
(Version VI, dated 4th September 2020). Accessed September 5,
2020. Available from:
https://www.icmr.gov.in/pdf/covid/strategy/Testing_Strategy_v6_04092020.pdf
28. Dinnes J, Deeks JJ, Adriano A, et al.
Rapid, point-of-care antigen and molecular-based tests for
diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev.
2020;8: CD013705.
29. Cochrane Gyanecology and Fertility group.
Excel sheet Perinatal outcomes in COVID-19 infection. Accessed 1
Sept, 2020. Available from:
https://cgf.cochrane.org/news/covid-19-coronavirus-disease-fertility-and-pregnancy
30. Centers for Disease Control and
Prevention. Evaluation and Management Considerations for
Neonates at risk for COVID-19. Accessed September 04, 2020.
Available from:
https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-newborns.html
31. Watson J, Whiting PF, Brush JE.
Interpreting a covid-19 test result. BMJ. 2020;369:m1808.
32. Ridgway JP, Pisano J, Landon E, Beavis
KG, Robicsek A. Clinical Sensitivity of Severe Acute Respiratory
Syndrome Coronavirus 2 Nucleic Acid Amplification Tests for
Diagnosing Coronavirus Disease 2019. Open Forum Infect
Dis.2020;7:ofaa315 [Published 2020 Jul 24].
33. Tang MS, Hock KG, Logsdon NM, et al.
Clinical performance of two SARS-CoV-2 serologic assays. Clin
Chem. 2020;66:1055-62.
|
|
|
 |
|

