|
|
|
Indian Pediatr 2020;57:1131-1134 |
 |
Detection of
Immunoglobulin M and Immunoglobulin G Antibodies Against
Orientia tsutsugamushi for Scrub Typhus Diagnosis and
Serosurvey in Endemic Regions
|
|
Mohan D Gupte, 1 Manish
Gupte,2 Suchit Kamble,3
Arati Mane,3 Suvarna Sane,3
Vijay Bondre,4
Jagadish Deshpande,5 Deepak
Gadkari6 and Manoj V
Murhekar7
From 1Indian Council of Medical Research, New Delhi; 2Independent
Scientist, Pune, India; 3ICMR-National AIDS Research Institute, Pune,
Maharashtra, India;4 ICMR-National Institute of Virology, Gorakhpur Unit,
Uttar Pradesh, India; 5ICMR-Enterovirus Research Centre, Mumbai,
Maharashtra, India; 6Independent Virologist, Pune, India;
7ICMR-National
Institute of Epidemiology, Chennai, Tamil Nadu, India
Correspondence to: Dr Manoj V Murhekar, ICMR-National Institute of
Epidemiology, Chennai, Tamil Nadu, India.
Email: [email protected]
Received: June 11, 2019;
Initial review: September 19, 2019;
Accepted: May 07, 2020.
Published online: September 07, 2020;
PII:
S097475591600242
|
Objectives: To estimate the regional cutoff of
optical density (OD) values for immuno-globulin M (IgM) antibodies
against Orientia tsutsugamushi in serum and cerebrospinal fluid
(CSF) for clinical diagnosis of scrub typhus and immunoglobulin G (IgG)
antibodies in serum for sero-epidemiology in Gorakhpur, Uttar Pradesh,
India. Methods: We used data from a serological investigation of
acute encephalitis syndrome patients (n=407) during the 2016
outbreak in Gorakhpur, India to determine the cutoff for OD values for
IgM antibodies, and from community-based serosurveys (n=1991) to
estimate the cutoff for OD values for IgG antibodies. Results: We
determined regionally relevant cutoff for OD values of 0.76 for IgM
antibodies in serum and 0.22 in cerebrospinal fluid for scrub typhus
diagnosis. For serosurveys, IgG antibody cutoff was 1.5. Conclusions:
We have proposed locally relevant cutoffs for scrub typhus endemic
regions, which may be useful for correctly classifying infected
population.
Keywords: Acute encephalitis syndrome, Diagnosis,
Epidemiology, Immunoassay.
|
|
S crub typhus, caused by
Orientia tsutsugamushi (OT), is the most common
re-emerging rickettsial infection in India and many other South
East Asian countries [1]. Although, various laboratory tests are
available for diagnosis of rickettsial infection, Enzyme-linked
immunosorbent assay (ELISA) based tests, particularly
immunoglobulin M (IgM) capture assays can be made available at
secondary and tertiary levels of healthcare [2]. The IgM ELISA
manufactured by InBios (Scrub Typhus Detect, InBios
International Inc., Seattle, USA) is considered as an
alternative to the gold standard immunofluorescent assay (IFA)
for diagnosis of acute infection [3].
IgM ELISA is meant for diagnostic purposes
only, whereas IgG antibodies indicate recent and/or past
exposure. IgG seroprevalence surveys are conducted to measure
endemicity of OT infection in an area. In India, InBios IgM
ELISA and IgG ELISA are commonly used for diagnosing scrub
typhus as well as measuring endemicity of infection [4-10]. The
manufacturer’s instructions recommend calculation of regional
cutoff for optical density (OD) values based on geographically
representative serum samples [11,12]. Moreover, the IgM assay is
recommended for diagnostic purposes using serum [11]; however,
its applicability in cerebrospinal fluid (CSF) is not known. The
present study was conducted to estimate the regional cutoff of
OD values for IgM antibodies against OT in serum and CSF
specimens for clinical diagnosis, and IgG antibodies in serum
for sero-epidemiology.
METHODS
For this study, we used data collected during
our previous two studies [5,13] in Gorakhpur, Uttar Pradesh,
India, a highly endemic area for scrub typhus. The first was on
407 inpatients (aged £14
years) with a clinical diagnosis of acute encephalitis syndrome
[14] during August to October, 2016. Blood and CSF samples were
available for serological investigations for 389 and 374
patients, respectively. Sera and CSF were tested for IgM
antibodies against OT using Scrub Typhus Detect ELISA. CSF was
diluted in 1:10 proportion for detection of IgM antibodies [5].
The other was data from two community-based serosurveys
conducted in Gorakhpur district to estimate prevalence of OT
infection [13]. These surveys were conducted among healthy
individuals in two separate groups of villages in Gorakhpur
district. Blood samples from 1991 individuals aged between 6 and
45 years were collected in these serosurveys, including 1085
during the phase-1 serosurvey, and 906 during the phase-2. Sera
were tested for IgG antibodies against OT using Scrub Typhus
Detect ELISAs following manufacturer’s instructions.
Statistical analyses: We used the
following methods for deciding the cutoff for OD values of IgM
and IgG antibodies against OT: (a) To determine the
cutoff for OD values of IgM antibodies against OT in serum, we
plotted the frequency distribution of OD values. The OD value
corresponding to the anti-mode was considered as the cutoff.
This method is often used for differentiating distribution of
infected and un-infected individuals in tuberculosis infection
surveys [15]. (b) We considered 356 AES patients for whom
both serum and CSF samples were available for analyses [5]. We
estimated the regression equation between OD values of IgM
anti-bodies against OT in serum and CSF. Using this equation, we
calculated the cutoff for OD value for CSF corresponding to the
cutoff for serum OD. (c) We plotted the frequency
distribution of OD values from healthy indivi-duals enrolled in
phase 1 and 2 of the serosurveys [13]. There was a bimodal
distribution, with segregation of values at the two ends and a
central portion of OD values close to the baseline. We
considered OD value corresponding to anti-mode of distribution
in phase-2 sero-survey and OD value corresponding to the
beginning of distribution of infected individuals in phase-1
survey as cutoffs.
RESULTS
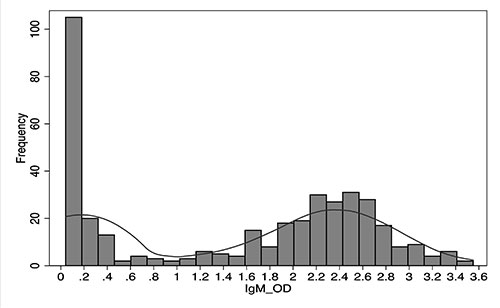
The frequency polygon of OD values of IgM
antibodies against OT in 389 AES patients showed a bimodal
distribution, with anti-mode at 0.76. This was considered as
cutoff for OD values against OT in serum IgM (Fig. 1).
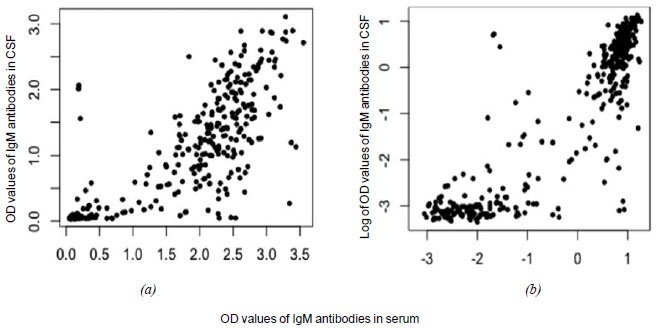
A scatter diagram for the paired observations for OD values of
IgM antibodies in serum and CSF showed a very strong positive
correlation with a correlation coefficient of 0.83 (95% CI
0.79-0.86) (Fig. 2). On linear regression
analysis, the relationship between the OD values of IgM
antibodies in serum and CSF was serum OD (Serum)=(1.07*CSF
OD)+0.52. Based on this equation, for OD value of 0.76 for IgM
antibodies in serum, the corresponding OD value for IgM
antibodies in CSF was 0.224.
 |
|
Fig. 1 Frequency distribution
of OD values for IgM antibodies against OT among 356 AES
patients, Gorakhpur, Uttar Pradesh, 2016.
|
 |
|
Fig. 2 Scatter diagram
showing (a) OD values and (b) log of OD values of IgM
antibodies against OT in CSF and serum (n= 356).
|
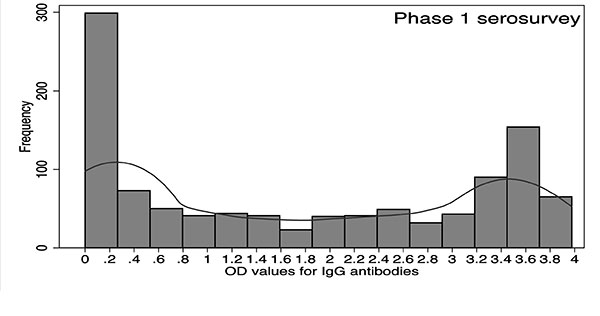
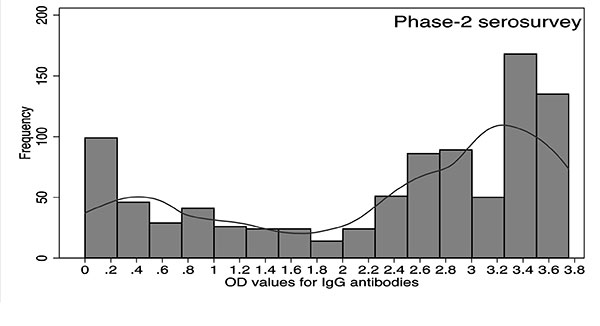
The distribution of OD values from phase-2
survey showed an anti-mode at 1.5; however, the distribution
from phase-1 survey did not reveal a clear demarcation between
infected and uninfected individuals. The central portion between
the two peaks was comparatively flat with OD values ranging from
0.6 to 2.5. OD value >2.5 in phase 1 corresponded to the
distribution of infected individuals. In phase-1 and phase-2
serosurveys, 155 (14.3%) and 133 (14.7%) observations were
between OD values of 1.5 and 2.5 (Fig. 3).
 |
| (a) |
(b) |
|
Fig. 3 Frequency distribution of OD values for
IgG antibodies against OT in (a) phase-1 and (b) phase-2
surveys, Gorakhpur, Uttar Pradesh, 2016.
|
DISCUSSION
Previous studies using Inbios ELISA kit have
used OD value of 0.5 as the cutoff for IgM as well as IgG
antibodies [4-10]. The manufacturer’s instructions recommend
calculation of cutoff value by determining the average of OD
plus three times of the standard deviation (SD) of sera from
healthy individuals and/or sera from persons with unrelated
infections. It is further recommended that the end users
calculate their cutoff using geographically relevant serum
samples [11,12].
The phase-1 and phase-2 serosurveys were
conducted among apparently healthy individuals at two different
periods of transmission of scrub typhus infection in the
community. However, using the OD values from children aged
£14
years, the cutoff for IgM antibodies as per the kit recommended
method, was 0.68 and 1.26 during the phase-1 and phase-2
surveys, respectively. The corresponding OD values for IgG
anti-bodies was >3 in both the surveys. Higher cutoff obtained
even during phase-1 survey, when the OT transmission in the
community is expected to be low, indicates that the population
included for sero-survey was not an unexposed population to OT.
In view of this, we decided to consider the distributions of OD
values for IgM among AES patients and IgG among healthy children
for finding out the optimal cutoff.
The cutoff for IgM antibodies determined by
us is higher than the cutoff of 0.5 observed by Blacksell, et
al. [3] but comparable to the cutoff of >0.8 identified in
another endemic area in India [18]. For IgG antibodies, A cutoff
OD value of ³1.5
in phase-1 serosurvey would have misclassified 14.3% individuals
as infected, while a cutoff OD value of
³2.5 in
phase-2 serosurvey would have misclassified 14.7% infected
individuals as unin-fected. Since the primary objective of
seroepidemio-logical studies is to estimate the disease burden,
certain amount of misclassification in unavoidable with either
cutoffs. The amount of misclassification; however, was not
different with either cutoff. We therefore suggest an OD value
of ³1.5
as cutoff for classifying individuals as infected with OT for
sero-epidemiological studies in Gorakhpur. With this cutoff, it
was still possible to see clear transition for OT infections
from 50.6% to 70.1% from phase-1 to phase-2 surveys. Trowbridge,
et al. [16] have recommended a cutoff of >1.8 for IgG
antibodies based on the community-based survey conducted in
another high endemic setting in India.
Although the kit is recommended for detecting
IgM antibodies only in serum samples, we observed good
correlation between OD values for IgM antibodies against scrub
typhus in serum and CSF. In comparison to serum where dilution
of 1:100 is used, for CSF we used dilution of 1:10, as
previously reported [17]. Since IgM antibodies cannot cross the
blood brain barrier, the presence of such antibodies in CSF
indicates that these antibodies are produced by antibody
secreting cells in the central nervous system and hence presence
of IgM antibodies against OT is more specific of scrub typhus
infection. The calculated cutoff for CSF would require further
evaluation before being used as a diagnostic criterion.
Our study has certain limitations. We did not
use any gold standard test to compare the performance of Inbios
ELISA to calculate the cutoff for IgM and IgG antibodies. It was
also not possible to calculate the cutoff based on manufacturer
recommended procedure of mean (3 SD) based on endemic normal
indivi-duals, in view of high endemicity of infection in the
area.
In conclusion, we have calculated regionally
relevant cutoffs for OD values of IgM in serum and CSF for
clinical diagnosis, as well as cutoff for OD values for IgG
antibodies for sero-epidemiological surveys in areas where OT
transmission is endemic. Further evaluation of these methods may
be used to find out accurate cutoffs in endemic areas, to
correctly classifying infected population.
Acknowledgement: Dr Rashmi Arora, Former
Head, Epidemiology and Communicable Diseases, Indian Council of
Medical Research, New Delhi for her support and technical inputs
on the study.
Contributors: MDG: conceived the study;
MDG, SK, MM: designed the study protocol and were involved in
sample/data collection; AM, SS, VB, JD: carried out laboratory
investigations; MG, MDG: analysed the data; MDG, MG, DG, MM:
inter-preted these data; MG, MDG: drafted the manuscript; DG,
MM: critically revised the manuscript for intellectual content.
All authors read and approved the final manuscript, and agree to
be accountable for; all aspects of the manuscript.
Ethical clearance: The
institutional ethics committee of National AIDS Research
Institute, Pune; No. NARI EC/2015-24 dated 13 August, 2015 and
NARI EC/2016-15 dated 12 September, 2015. National Institute of
Epidemiology, Chennai; No.NIE/IHEC/201507/-01 and dated 20 July,
2016.
Funding: Indian Council of Medical
Research, New Delhi; Competing interest: None stated.
REFERENCES
1. Rajapaksea S, Weeratungab P,
Sivayoganathana S, Fernandoc SD. Clinical manifestations of
scrub typhus. Trans Roy Soc Trop Med Hyg. 2017;111:43-54.
2. Department of Health Research – Indian
Council of Medical Research. Guidelines for diagnosis and
management of rickettsial diseases in India. 2013. Available
from:
https://www.icmr.nic.in/sites/default/files/guidelines/DHR-ICMR%20Guidelines%20on%20
Ricketesial%20Diseases. pdf. Accessed February 14, 2020.
3. Blacksell SD, Tanganuchitcharnchai A,
Nawtaisong P, et al. Diagnostic accuracy of the in bios
scrub typhus detect enzyme-linked immunoassay for the detection
of IgM antibodies in Northern Thailand. Clin Vaccine Immunol.
2015;23:148-54.
4. Murhekar MV, Mittal M, Prakash JA, et
al. Acute encephalitis syndrome in Gorakhpur, Uttar Pradesh,
India - Role of scrub typhus. J Infect. 2016;73:623-26.
5. Mittal M, Bondre V, Murhekar M, et al.
Acute encephalitis syndrome in Gorakhpur, Uttar Pradesh, 2016:
Clinical and laboratory findings. Pediatr Infect Dis J.
2018;37:1101-06.
6. Thangaraj JWV, Vasanthapuram R, Machado L,
et al. Risk factors for acquiring scrub typhus among
children in Deoria and Gorakhpur districts, Uttar Pradesh,
India, 2017. Emerg Infect Dis. 2018;24:2364-67.
7. Bal M, Mohanta MP, Sahu S, Dwibedi B, Pati
S, Ranjit M. Profile of pediatric scrub typhus in Odisha, India.
Indian Pediatr. 2019;56:304-06.
8. Morch K, Manoharan A, Chandy S, et al.
Acute undifferentiated fever in India: A multicentre study of
etiology and diagnostic accuracy. BMC Infect Dis. 2017;17:665..
9. Bhargava A, Kaushik R, Kaushik RM, et
al. Scrub typhus in Uttarakhand and adjoining Uttar Pradesh:
Seasonality, clinical presentations and predictors of mortality.
Indian J Med Res. 2016;144:901-9.
10. Kalal BS, Puranik P, Nagaraj S, Rego S,
Shet A. Scrub typhus and spotted fever among hospitalized
children in India: Clinical profile and serological
epidemiology. Indian J Med Microbiol. 2016;34:293-8.
11. Scrub Typhus Detect IgM ELISA System.
Available from:http://www.inbios.com/wp-content/uploads/2016/06/Scrub-Typhus-ELISA-and-Rapid-develop.-05.16.pdf.
Accessed Febuary 14, 2020.
12. Scrub Typhus Detect IgM ELISA System.
Available from:
http://www.diatek.in/inbios/Scrub_Typhus_Detect_ IgG.pdf.
Accessed February 14, 2020.
13. Kamble S, Mane A, Sane S, et al.
Seroprevalence and Seroincidence of O. tsusugamushi infection in
Gorakhpur, Uttar Pradesh, India: A community based serosurvey
during lean (April-May) and epidemic (October-November) periods
for acute ence-phalitis syndrome. Indian J Med Res.
2020;151:350-60.
14. World Health Organization. Acute
Encephalitis Syndrome. Japanese Encephalitis Surveillance
Standards. January, 2006. In: WHO-recommended standards
for surveillance of selected vaccine-preventable diseases.
WHO/V&B/03.01. Available from: http://apps.who.int/iris/bitstream/10665/68334/1/WHO_V-B_03.01_eng.pdf.
Accessed February 14, 2020.
15. Styblo K. Recent advances in
epidemiological research in tuberculosis. Adv in Tuberc Res.
1980;20:1-63.
16. Trowbridge P, Divya P, Premkumar PS,
Varghese GM. Prevalence and risk factors for scrub typhus in
South India. Trop Med Int Health. 2017;22:576-82.
17. Burke S, Nisalak A, Ussery MA. Antibody
capture immunoassay detection of Japanese encephalitis virus
immunoglobulin M and G antibodies in cerebrospinal fluid, J Clin
Microbiol. 1982;16:1034-42.
|
|
|
 |
|

