|
|
|
Indian Pediatr 2020;57:
1110-1113 |
 |
Pediatric Liver Transplantation in India: 22
Years and Counting
|
|
Smita Malhotra, 1 Anupam
Sibal1
and Neerav Goyal2
From 1Department of Pediatric Gastroenterology and Hepatology and
2Hepatobiliary and Pancreatic Surgery Unit, Indraprastha Apollo
Hospitals, New Delhi, India.
Correspondence to: Dr Smita Malhotra, Department of Pediatric
Gastroenterology and Hepatology, Apollo Institute of Pediatric Sciences,
Indraprastha Apollo Hospital, New Delhi, India.
Email:
[email protected]
|
|
Liver transplantation in
India has grown exponentially in the last decade with 135
centers now performing between 1500-2000 transplants a year, 10%
of which are pediatric. Survival rate surpassing 90% has been
achieved, and India is now an important regional liver
transplant hub in South and South-East Asia. The indications
have expanded to include increasing number of liver-based
metabolic disorders that may or may not cause liver disease.
Recipients, who were previously considered non-transplantable
such as those with pre-existing portal vein thrombosis, can be
successfully managed with innovative microvascular techniques.
The donor pool has grown with the use of marginal grafts and ABO
incompatible organs. Financial constraints are being overcome by
crowd funding and increasing philanthropic efforts.
Keywords: Deceased donor, History,
Multi-organ transplant, Outcome.
|
|
L iver transplant is curative
for acute liver failure,
chronic end stage liver diseases, some liver
tumors and inborn metabolic errors that may or may not cause liver
disease per se. Advancements in preoperative care,
surgical techniques, intensive care management along with availability
of potent immunosuppressive drugs have helped attain 94% 1-year, 91%
5-year, and 88% 10-year patient survival in pediatric liver transplant
recipients [1]. In India, the first successful pediatric liver
transplant was performed in 1998 [2], and that boy with biliary atresia
is now about to complete his graduation in medicine and is doing well on
minimal immunosuppression (Table I). Every year around
1500-2000 liver transplants are now performed, of which approximately
10% are pediatric. Survival rates sur-passing 90% have since been
attained in India [3-5]. Initial progress was slow as part of a learning
curve with limitations due to scarcity of trained personnel, poor
awareness amongst primary care doctors, reservations regarding donor
safety and the financial implications. By 2007, only 318 liver
transplants had been performed in India [6]. The growth has been
exponential in the last decade; although collated data of the country is
not available. This has been sustained mainly by living donor liver
transplant (LDLT) though deceased donation (DDLT) is picking up,
primarily in the southern part of the country where some centers report
a 70/30 LDLT/DDLT overall distribution [7]. As per the Global
Observatory on Donation and Transplantation, 1945 liver transplants
(adult and pediatric) were performed in India in the year 2018 (1313
LDLT).
Table I History of Pediatric Liver Transplantation in India
| Event |
Year |
| Pediatric living donor liver transplant
(LDLT) |
1998 |
| Adult deceased donation liver
transplant (DDLT) |
1998 |
| Liver transplant for acute liver
failure (ALF) |
1999 |
| Adult combined liver and kidney
transplant |
1999 |
| Pediatric combined liver and kidney
transplant |
2007 |
| Pediatric re-transplant |
2002 |
| Pediatric DDLT |
2007 |
| Liver transplant for HIV |
2008 |
|
Liver transplant for Criggler-Najjar syndrome
|
2008 |
| Domino transplant |
2009 |
| LDLT for factor VII deficiency |
2010 |
| Auxillary liver transplant for
ALF |
2012 |
| ABO incompatible liver transplant |
2014 |
|
Domino auxillary liver transplant |
2015 |
Auxiliary liver transplant (auxiliary partial
orthotopic liver transplant, APOLT) with implantation of a partial graft
without fully removing the native liver is technically more challenging
with a higher complication rate. It is a suitable option for acute liver
failure and metabolic disorders without cirrhosis as it offers a chance
for immunosuppression free life in case of native liver regeneration or
restoration of defective metabolic function with newer therapies.
Successful APOLT for acute liver failure has been reported from India
but the modality has not become popular due to the higher risks involved
with retaining a diseased liver in acute liver failure with its
attendant toxic and metabolic effects.
The establishment of a new liver transplant program
requires approval from a regional health body after evaluation of
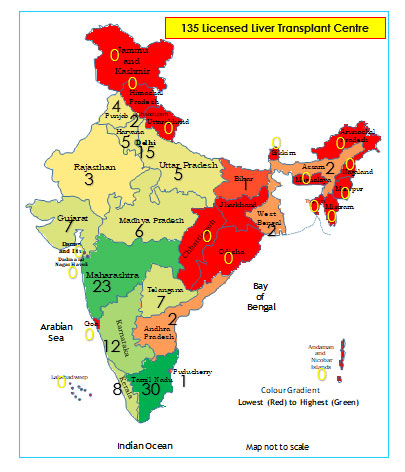
infrastructure and expertise. As per the National organ and tissue
transplant organisation (NOTTO) there are now about 135 centers for
liver trans-plant in India, including second tier cities apart from the
metros, with majority of the pediatric work limited to about 10 centers
(Fig. 1). As liver transplant is largely private sector
driven and is a high cost procedure, credibility rests on preventing
commercialization of the programs. This has been made feasible by the
stringiest criteria laid down by the government and strict scrutiny by
authorization committees in every case to ensure donation is from a
relative on a wholly voluntary basis with no coercion. In camera
meetings are held and all documents to establish near relationship are
verified before a go ahead is attained even in cases requiring emergency
transplants.
 |
|
Fig. 1 Distribution of liver
transplantation centers in India, 2020.
|
A significant proportion of pediatric patients are
from overseas. Patients from about 20 countries have been transplanted
at our center, and also at other Indian centers. A few are partially or
wholly funded by their governments, while many others raise funds
through international charities and/or social organizations. Many
families have gone on to form support groups in their respective
countries. The Indian Human Organ Trans-plant Act, enacted in 1994,
allows foreign nationals to receive a deceased organ in India only if no
suitable Indian recipient is available. As cadaveric donations are few
and the waiting list is long, foreign nationals would only qualify for
LDLT.
India is now an important regional center for liver
transplant in South and South East Asia, more so for pediatric patients.
Many of these neighboring countries have either not yet set up
transplant units or are in the fledgling phase running predominantly
adult programs. Pediatric liver transplant carries its own set of
challenges due to the smaller diameter of vessels requiring greater
surgical expertise along with need for specialized pediatric intensive
care and usually a longer duration of postoperative hospital stay.
Moreover, many of these countries have very few pediatric hepatologists
with expertise in post-transplant care, thus necessitating a thorough
coordination with the transplant unit for follow up once the families
travel back to their native countries.
EXPANDING THE DONOR AND RECIPIENT POOL
Though biliary atresia remains the leading indication
for liver transplant in children [8], improving outcomes have encouraged
expanding indications to include liver-based metabolic defects [9].
Where these defects cause liver damage, liver transplant is curative by
replacing the diseased organ as in other disorders of liver architecture
leading to synthetic dysfunction and decompensation. The other group
includes disorders whereby a genetically inherited enzymatic defect,
wholly or partially liver based with a structurally normal liver, causes
neurological/multiorgan involvement that may be prevented by replacing
the liver. Liver transplant should be performed early before
irreversible damage occurs in target organs. These include Criggler
Najjar syndrome, urea cycle defects and organic acidemias to achieve
intact neuro-logical status, familial hypercholesterolemia to prevent
cardiac disease and/or sudden death, and primary hyperoxaluria where an
early liver transplant prevents renal failure or else a
combined/sequential liver kidney transplant would be required to prevent
systemic oxalate overload and its multiorgan consequences. With the
availability of next generation sequencing (NGS) based tests, precise
and timely genetic diagnosis can now be made and timely therapy
instituted.
Livers from patients with Maple syrup urine disease
(MSUD) and familial hypercholesterolemia may be donated to cirrhotic
patients as they are structurally and functionally normal apart from an
enzyme deficiency, which may be compensated by other body tissues to
sustain function. Such transplants, known as domino transplants, have
been successfully reported from India [10]. As our programs are
primarily living-related, the majority of the donors are parents who are
carriers of the recipients’ metabolic or genetic disorders with
autosomal recessive inheritance. Use of such heterozygous donors has
been debated. Data from the Japanese multicenter registry [11] has
reported that outcome after employing heterozygous donors was excellent
with better long-term survival rate. Portal vein thrombosis (PVT), a
known sequelae of cirrhosis, especially more frequently seen in infants
with biliary atresia due to portal vein hypoplasia, is no longer a
contraindication to liver transplant despite the technical difficulty
and higher risks of post-transplant vascular thrombosis endangering the
graft [12].
Pediatric liver transplant has now progressed beyond
the ABO blood group barrier. ABO incompatible (ABOi) transplants are
being increasingly performed when blood group compatible donor from the
family is not available. Despite concerns about liver graft
regeneration, antibody mediated rejection (AMR), higher incidence of
biliary strictures and sepsis, both pediatric and adult ABOi liver
transplant survival has improved markedly and has become comparable to
ABO-compatible liver transplant with the introduction of rituximab
prophylaxis before transplant [13]. Rituximab and/or other B cell
desensiti-zation strategies including plasmapheresis and IVIg are used
to bring down isoagglutinin titers to less than 1/8 pre liver
transplant. Children younger than 2 years of age may not require these
desensitization therapies as blood group isoagglutinins titers are low
and complement system activation is not robust, thus minimizing the
risks of AMR [14]. ABOi liver transplant grafts in acute liver failure
in infants have thus been used more often as lack of time window for
desensitization strategies may limit use of this modality in older
children and adults with acute liver failure [15]. Most busy centers in
India have successfully performed ABOi transplants since the initial
reports of success in 2014 [16].
Size-matching determined by the graft-to-recipient
weight ratio (GRWR) is a crucial determinant for graft suitability and
ideal ratios of 0.8-1 have been advocated. Many centers now have
experience with small for size grafts and it is acceptable for the GRWR
to be as low as 0.5-0.6 if there are accompanying factors of portal vein
pressure £15
mmHg, middle hepatic vein reconstruction, or young donor age [17]. On
the other hand, large for size grafts are problematic in small infants
due to compro-mised portal venous flow and small abdominal cavity. Use
of reduced mono/bi-segment grafts and delayed abdominal closure using
mesh/skin closure help circum-vent abdominal compartment syndrome [16].
Thus, babies as small as 4-5 kg are now being routinely transplanted at
select centers in India.
Other marginal grafts are increasingly being
accepted. These include older donors in the absence of size mismatch and
severe steatosis, moderately steatotic liver grafts if predominant
pattern is of microsteatosis instead of macrosteatosis, donors with a
BMI ³30 kg/m2
and HBsAg-negative/HBcAb-positive liver grafts in HBsAg negative
recipients with active immunization and post-transplant antiviral
prophylaxis to prevent de novo HBV infection [17]. These
strategies have considerably increased the donor pool for liver
transplant in scenarios hitherto found unsuitable.
PERSISTING CONCERNS
Studies on long term morbidities, effects of immuno-suppression
and quality of life post liver transplant are lacking from our country.
LT requires lower immuno-suppression compared to other organs. Indian
patients have been shown to do well on lower immunosuppression as
infections are more common in our scenario [4,5]. Regimens vary across
different programs but cortico-steroids remain the induction agents of
choice with dual agent regimens including calcineurin inhibitors and
renal sparing mycophenolate for the first year, with the aim to come
down to a single agent by the second year. The desired ideal outcome is
attainment of prope tolerance, i.e., almost immune tolerant state
where the recipient is alive with first allograft with no ongoing
rejection episode on tacrolimus therapy with trough levels less than 3
ng/mL, three years post LT. Studies indicate that almost 20% pediatric
patients may attain such immune tolerance with maximal chances for those
transplanted in infancy [18]. However, the SPLIT database analysis has
revealed that the ideal triad of normal growth, stable allograft
function on single-agent immunosuppression, and an absence of
immunosuppression-related complications is achieved in only about a
third of recipients 10 years after LT [1]. Steroid free protocols using
antibody induction (ATG/basiliximab) along with tacrolimus are not yet
in vogue but steroid-free tacrolimus-based immuno-suppression may result
in an enhancement of graft acceptance in the long term as well as in a
higher proportion of children becoming prope tolerant [19,20].
Lack of a database greatly inhibits accurate analysis
of trends, outcomes and long term results. Non-availability of long
term followup from many recipients from overseas is another
disadvantage. With the formation of the Liver Transplant Society of
India, efforts are on for a national registry, hopefully more data
should be available in the coming years. Low numbers for DDLT,
especially in Northern India, is amongst the foremost immediate concern
that requires intense campaigning and education to change the social
mindset. Encouraging organ donation is the need of the hour.
CHANGING SOCIAL SCENARIO
Perhaps, the most crucial limiting factor in our
country has been the cost of liver transplant. The programs are largely
driven through the private sector, and health care insurance is still
not widely prevalent in our country. Moreover, most insurance companies
do not provide cover for diseases of perinatal onset or genetic etiology.
The advent of crowd funding platforms where strangers come together on
the internet to fund a medical catastrophe for an unknown person is
heart-warming and provides an insight into the social responsibility the
community is prompt to take up when transparency is assured. These
campaigns run on social media with tight timelines ranging from a week
to a month, and at times funds have been raised in a day or two for
emergency transplants. Crowd funding with the support of few
philantrophic organizations and individuals dedicated to funding liver
transplants has thus made transplantation attainable for those with
limited resources. Crowd funding works best for children awaiting
trans-plants, perhaps due to the emotive pull of images and videos of
innocent children struggling to wade off certain death that a transplant
could prevent. The predicament of the parents, one of whom is the organ
donor most of the time, also touches a cord. With this active support of
the community in facilitating transplants for children, liver transplant
seems to have finally come of age in our country.
FUTURE DIRECTIONS
High-resolution sequence mapping of DNA variation is
now feasible and liver tissue transcriptional signatures are being
studied to identify candidates likely to achieve tolerance and
withdrawal of immune suppression. Genome wide association studies or NGS
for cytokine genotyping to detect single nucleotide polymorphisms in
cytokine gene promotor regions may help identify recipients at low risk
for rejection.
Meanwhile, expansion of this facility in the public
sector is needed as liver transplant is still not available routinely to
those with limited resources. We wait to realize the dream of DDLT
becoming the primary modality, as it is in the Western countries, by
concerted efforts to promote organ donation.
REFERENCES
1. Ng VL, Alonso EM, Bucuvalas JC, et al., for
Studies of Pediatric Liver Transplantation (SPLIT) Research Group.
Health status of children alive 10 years after pediatric liver
transplantation performed in the US and Canada: Report of the studies of
pediatric liver transplantation experience. J Pediatr. 2012;160:820-26.
2. Poonacha P, Sibal A, Soin AS, Rajashekar MR, Raja-kumari
DV. India’s first successful pediatric liver trans-plant. Indian Pediatr.
2001;38:287-91.
3. Sibal A, Bhatia V, Gupta S. Fifteen years of liver
transplan-tation in India. Indian Pediatr. 2013;50:999-1000.
4. Mohan N, Karkra S, Rastogi A, et al.
Outcome of 200 pediatric living donor liver transplantation in India.
Indian Pediatr. 2017;54:913-19.
5. Sibal A, Malhotra S, Guru FR, et al.
Experience of 100 solid organ transplants over a five year period from
the first successful pediatric multi organ transplant program in India. Pediatr
Transplant. 2014;18:740-5.
6. Kakodkar R, Soin A, Nundy S. Liver transplantation
in India: its evolution, problems and the way forward. Natl Med J India.
2007;20:53-56.
7. Narasimhan G, Kota V, Rela M. Liver
transplantation in India. Liver Transpl. 2016;22:1019-24
8. Malhotra S, Sibal A, Bhatia V, et al.
Living related liver transplantation for biliary atresia in the last 5
years: Experience from the first liver transplant program in India.
Indian J Pediatr. 2015:82:884-9.
9. Mazariegos G, Shneider B, Burton B, et al.
Liver trans-plantation for pediatric metabolic disease. Mol Genet Metab.
2014;111:418-27.
10. Mohan N, Karkra S, Rastogi A, Vohra VK, Soin AS.
Living donor liver transplantation in maple syrup urine disease. Case
series and world’s youngest domino liver donor and recipient. Pediatric
Transplant. 2016;20: 395-400.
11. Kasahara M, Sakamoto S, Horikawa R, et al.
Living donor liver transplantation for pediatric patients with metabolic
disorders: the Japanese multicenter registry. Pediatr Transpl.
2014;18:6-15.
12. Conzen KD, Pomfret EA. Liver transplant in
patients with portal vein thrombosis: medical and surgical requirements.
Liver Transpl. 2017;23:S59-S63.
13. Yamamoto H, Uchida K, Kawabata S, et al.
Feasibility of monotherapy by rituximab without additional
desensiti-zation in ABO-incompatible living-donor liver
trans-plantation. Transplantation. 2018;102:97-104.
14. Honda M, Sugawara Y, Kadohisa M, et al.
Long-term outcomes of ABO-incompatible pediatric living donor liver
transplantation. Transplantation. 2018;102:1702 09.
15. Yamamoto H, Khorsandi SE, Cortes Cerisuelo M, et
al. Outcomes of liver transplantation in small infants. Liver
Transpl. 2019;25:1561-70.
16. Soin AS, Raut V, Mohanka R, et al. Use of
ABO-incom-patible grafts in living donor liver transplantation – First
report from India. Indian J Gastroenterol. 2014;33:72-6.
17. Lan X, Zhang H, Li HY, et al. Feasibility
of using marginal liver grafts in living donor liver
transplantation. World J Gastroenterol. 2018;24:2441-56.
18. Mazariegos GV, Sindhi R, Thomson AW, Marcos A.
Clinical tolerance following liver transplantation: Long term results
and future prospects. Transpl Immunol. 2007; 17: 114-19.
19. Gras JM, Gerkens S, Beguin C, et al.
Steroid-free, tacro-limus-basiliximab immunosuppression in pediatric
liver transplantation: Clinical and pharmacoeconomic study in 50
children Liver Transpl. 2008;14:469-77.
20. Bourdeaux C, Pire A, Janssen M, et al.
Prope tolerance after pediatric liver transplantation. Pediatr Transpl.
2013; 17:59-64.
|
|
|
 |
|

