|
|
|
Indian Pediatr 2017;54: 1017-1020 |
 |
Double Dose Versus
Standard Dose Hepatitis B Vaccine in HIV-infected Children:
A Randomized Controlled Trial
|
|
Shahid Akhtar Siddiqui, Manisha Maurya, DK Singh,
Anubha Srivastava and Ruchi Rai
From Department of Pediatrics, MLN Medical College,
Allahabad, Uttar Pradesh, India.
Correspondence to: Prof Ruchi Rai, A-77, Sector 21,
Jalvayu Vihar, NOIDA 201303, Uttar Pradesh, India.
Email: [email protected]
Received: December 13, 2016;
Initial Review: March 02, 2017;
Accepted: July 25, 2017.
Published online:
August 24, 2017.
Trial Registration: CTRI/2016/01/006495
PII:S097475591600088
|
|
Objective: To compare the
efficacy of double dose (20 µg) with standard dose (10 µg) of hepatitis
B vaccine in HIV-infected children. Methods: Unvaccinated
HIV-infected children were randomized to receive 3 doses of double dose
(N=27) or standard dose (N=28) of recombinant Hepatitis B
vaccine. Anti-HBs antibody titres were measured 3 mo after the last
dose. An antibody titre ł10
mIU/mL 12 weaks after the third dose was considered as serporotection.
Result: Seroprotection was achieved by 17 (60.7%) children in
standard dose group against 20 (74%) in the double dose group [RR
(95%CI) 0.8 (0.17-1.7); P=0.29]. CD4 count < 500 cells/mm3 was
significantly associated with lower rates of seroprotection.
Conclusion: Double dose of hepatitis B vaccine does not seem to
provide any advantage when compared to standard dose in HIV-infected
children.
Keywords: Immunization, Immunodeficiency,
Prevention, Vaccination.
|
|
C
o-infection with viruses like Hepatitis B and C
is common in HIV-infected children [1]. All HIV-infected children must
therefore be vaccinated against hepatitis B. Multiple factors lead to
suboptimal response following vaccination in these children [2,3]. Even
HIV-exposed but uninfected infants have been shown to have an altered
immune response to vaccination [3,4]. This raises concern regarding the
appropriate dose and schedule of vaccines to be administered to these
children in order to achieve seroprotection. Numerous studies have shown
a much lower level of seroprotection with Hepatitis B vaccine (HBV) in
HIV-infected children and adults [5,6]. Various strategies to improve
the seroconversion rates – like higher dose of the vaccine, additional
doses of the standard dose or revaccination of the non-responders either
by the double dose or standard dose [7,8] – have been tried. There is
scarcity of data on seroconversion to HBV in HIV-infected Indian
children on highly active antiretroviral therapy (HAART).
We conducted this study to compare the efficacy of
double dose and standard dose of HBV in HIV-infected children.
Methods
The study was a parallel group randomized controlled
trial conducted at Anti retroviral therapy (ART) center of a tertiary
level teaching hospital in Allahabad, UP, India from August 2014 to July
2015. The study was approved by the Institutional Ethical Committee
(IEC). Written informed consent was obtained from the
parents/grandparents.
HIV-infected children in the age group between 18
months and 18 years fulfilling the following criteria were enrolled for
the study: (i) Unvaccinated for Hepatitis B in the past and (ii)
HBsAg negative. Children who were critically ill at the time of
enrolment or anytime during the study were excluded from the study. The
primary outcome measure was the Anti-HBs antibody titers 12 weeks after
the 3rd dose of HBV.
All eligible children were randomized into Standard
dose or Double dose groups with an allocation ratio of 1:1 using block
randomization with blocks of 6 (www.randomizer.org). Children
assigned to the standard dose group were given 0.5 mL (10 µg) of
recombinant HBV deep intramuscular at 0, 1, 6 months. Children assigned
to the double dose group were given 1 mL (20 µg) of HBV in the same
schedule. Allocation was concealed in sequentially numbered, opaque and
sealed envelopes, which were opened when a child was enrolled. All the
children were thoroughly assessed before enrolment and a detailed
history was taken. The children were classified according to the revised
World Health Organization (WHO) clinical staging and WHO immunological
staging. The children received HAART according to the existing National
AIDS Control Organization (NACO) guidelines. Anti-HBs antibody titres
were estimated using enzyme linked immuno-sorbent assay (ELISA)
(DS-EIA-ANTI-HBs) kit 12 weeks after the 3rd dose of HBV. Anti HBs titre
ł10 mIU/mL
were considered as seroprotection.
Statistical analysis was done using Epi info 7
software. The data of the two groups were compared using the Chi square
test, Student’s t test or Mann- Whitney U test.
Results
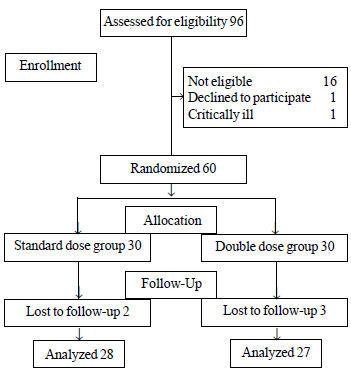
A total of 60 children were enrolled in the study
with final analysis of 55 children (Fig. 1). The baseline
characteristics were comparable in both the groups (Table I).
Seroprotection was achieved by 17 (60.7%) children in standard dose
against 20 (74%) in double dose group but it was not statistically
significant (Table II). There was no difference in the
seroprotective levels achieved when the children in both the groups were
further stratified into two subgroups based on the CD4 counts at the
time of enrolment; CD4 count <500 cells/mm 3
and CD count ł500
cells/mm3. CD4 count <500/mm3
was independently associated with significantly lower rates of
seroprotection irrespective of the dose of the vaccine (P=0.008)
(Table II). The coefficient of correlation (r) between the
CD4 count and the Anti HBs titers achieved was 0.31 (P<0.001)
showing a weak linear positive correlation.
TABLE I Baseline Characteristics in Study Children
|
Standard dose (n=28) |
Double dose(n=27) |
|
M:F |
3:1 |
2.3:1 |
|
Age (no.) |
|
|
|
18 mo - 5 y |
3 |
1 |
|
5-10 y |
12 |
15 |
|
10-18 y |
13 |
11 |
|
Children on HAART |
25% |
22.2% |
|
#*CD4 count (/mm3) |
719.8 (288.9) |
730 (396.5) |
|
*at enrolment; #values in mean (SD). |
 |
|
Fig.1 Study flow chart.
|
TABLE II Comparison of the Outcomes in Study Groups
|
SD (N=28) |
DD (N=27) |
RR (95% CI) |
P Value |
|
Seroprotected, N(%) |
17 (60.8%) |
20 (74%) |
0.8 (0.17,1.7) |
0.29 |
|
CD4 <500/mm3 |
3/8 (37.5%) |
3/7 (42.8%) |
0.87 (0.2, 3.0) |
0.62 |
|
CD4 ≥500mm3 |
14/20 (70%) |
17/20 (85%) |
0.8 (0.5, 1.1) |
0.22 |
|
*Anti HBs titer (mIU/mL) |
42.5 (7.5-335) |
370 (9-1145) |
|
0.09 |
|
*Median (IQR); SD standard dose; DD double dose. |
TABLE III Characteristics of Seroprotected and Unprotected Group
|
Achieved seroprotection |
Not achieved seroprotection |
RR (95% CI) |
P Value |
|
ART/No ART (N) |
6/31 |
7/11 |
1.59 (0.8-2.95) |
0.06 |
|
CD4 count, Mean (SD) (/mm3) |
788.02 (328.62) |
596.16 (350.18) |
|
0.05 |
|
CD4 count |
|
<500/mm3 (N=15) |
6 (40%) |
9 (60%) |
0.51 (0.27-0.98) |
0.008 |
|
≥500/mm3 (N=40) |
31 (77.5%) |
9 (22.5%) |
|
|
Discussion
In this study comparing the efficacy of double dose
and standard dose HBV vaccine in HIV-infected children, the
seroprotection rate in the double dose group was 74% compared to 60.8%
of the standard dose group, but it was not statistically significant.
The CD4 count at the time of enrolment was significantly associated with
seroprotection with a linear positive relationship.
The limitation of the study is the small sample size
because of the limited period of the study and single center-based
enrolment. Only about one-fourth of these children were receiving HAART.
Long-term follow-up for duration of seroprotection or development of
hepatitis B infection was also not done in the present study.
Suboptimal immunological response to HBV in
HIV-infected patients has been documented by numerous studies. A search
for the ideal dose and schedule for the HBV in such individuals has not
lead to a final consensus. Psevdos, et al. [9] studied the
efficacy of double dose of HBV in HIV-infected individuals who failed to
respond to standard dose vaccination. The double dose was compared with
additional standard doses in non-responders. The response rate was
significantly higher in the double dose group (85%) vs.
additional standard doses (61%). Cornejo Juarez, et al. [10]
conducted a randomized controlled trial comparing 10 µg dose with 40 µg
dose and found no significant difference. Fonseca, et al. [11]
found no significant difference in response to double dose of HBV in
HIV- infected adults with seroconversion rates 47% compared to 34% in
standard dose. However, double dose showed significantly higher response
in individuals with CD4 ł350
cells/mm3 and HIV viral load
<10,000 copies/mL. A meta-analysis by Ni, et al. [7] concluded
that the response rates in the patients who received high dose was
higher (OR 1.96; 95% CI 1.47, 2.61) [7].
A study by Pasricha, et al. [12] in India
found significantly lower HBsAb levels in HIV-infected patients,
especially those with a low CD4 count (<200 cells/mm 3),
even with a double dose when compared to standard dose administered to
healthy subjects. Bose, et al. [8] studied the immune response to
4 doses of double dose vaccine in HIV-infected children and found high
(94%) seroconversion.
We found a CD4 count of <500 cells/mm 3
to be associated with significantly poor immune response. Other studies
also found significantly suboptimal immune response in patients with a
low CD4 count [13,14]. The use of ART did not significantly affect the
immunological response of children in the index study. Cornejo-Juárez,
et al. [9] found no association between type and duration of
HAART and seroconversion but Psevdos, et al. [10] found use of
HAART to be significantly associated with seroconversion.
We conclude that double dose of HBV does not seem to
lead to higher seroprotection rate than standard dose in HIV-infected
children. Further studies with a larger sample size and stratified
according to age and CD4 counts will help us in understanding the need
of modifying the dose of HBV in HIV-infected children in a better way.
Contributors: SAS, DKS: involved in
designing the study, analysis of data and writing the manuscript; SAS,
MM: involved in data collection and analysis; RR, AS: were involved in
critical evaluation of the manuscript and analysis.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
• Three doses of 20 µg of hepatitis B
vaccine do not seem to offer significantly higher rate of
seroprotection than standard dose (10 µg) in HIV- infected
children (aged >18 months) on anti-retroviral therapy.
|
References
1. Kourtis AP, Bulterys MJ, Hu D, Jamieson DJ.
HIV–HBV coinfection – A global challenge. N Engl J Med. 2012; 366:
1749-52.
2. Yao ZQ, Moorman JP. Immune exhaustion and immune
senescence two distinct pathways for HBV vaccine failure during HCV
and/or HIV infection. Arch Immunol Ther Exp. 2013;61:193-201.
3. Njom Nlend AE, Nguwoh PS, Ngounouh CT, Tchidjou
HK, Pieme CA, Otele JM, et al. HIV-infected or -exposed children
exhibit lower immunogenicity to hepatitis B vaccine in Yaounde,
Cameroon: An appeal for revised policies in tropical settings? PLoS One.
11: e0161714.
4. Singh DK, Kumar R, Rai R, Maurya M, Bhargava A.
Immunogenicity of hepatitis B vaccine in HIV-infected exposed uninfected
infants. Indian J Pediatr. 2016; 83:172-4.
5. Abzug MJ, Warshaw M, Rosenblatt HM, Levin MJ,
Nachman SA, Pelton SI. Immunogenicity and immunologic memory after
hepatitis B virus booster vaccination in HIV-infected children receiving
highly active antiretroviral therapy. J Infect Dis. 2009; 200:935-46.
6. Zuin G, Principi N, Tornaghi R, Paccagnini S, Re
M, Massironi E, et al. Impaired response to hepatitis B vaccine
in HIV infected children. Vaccine. 1992;10:857-60.
7. Ni JD, Xiong YZ, Wang XJ, Xiu LC. Does increased
hepatitis B vaccination dose lead to a better immune response in
HIV-infected patients than standard dose vaccination: a meta-analysis?
Int J STD AIDS. 2013;24:117-22.
8. Bose D, Chandra J, Dutta R, Jais M, Ray S, Gupta
RA, et al. Immune response to double dose hepatitis-B vaccine
using four dose schedule in HIV infected children. Indian J Pediatr.
2016;83:772-6.
9. Psevdos G, Kim JH, Groce V, Sharp V. Efficacy of
double-dose hepatitis B rescue vaccination in HIV-infected patients.
AIDS Patient Care STDS. 2010;24:403-7.
10. Cornejo-Juárez P, Volkow-Fernández P, Escobedo-López
K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramírez LE. Randomized
controlled trial of Hepatitis B virus vaccine in HIV-1-infected patients
comparing two different doses. AIDS Res Ther. 2006:3:9.
11. Fonseca MO, Pang LW, de Paula Cavalheiro N,
Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B
vaccine in HIV-infected adult patients comparing a standard dose to a
double dose. Vaccine. 2005; 23:2902-8.
12. Pasricha N, Datta U, Chawla Y, Singh S, Arora SK,
Sud A, et al. Poor responses to recombinant HBV vaccination in
patients with HIV infection. Trop Gastroenterol. 2005;26:178-82.
13. Rey D, Krantz V, Partisani M, Schmitt MP, Meyer
P, Libbrecht E, et al. Increasing the number of hepatitis B
vaccine injections augments anti-HBs response rate in HIV-infected
patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161-5.
14. Pettit NN, DePestel DD, Malani PN, Riddell J 4th. Factors
associated with seroconversion after standard dose hepatitis B
vaccination and high-dose revaccination among HIV-infected patients. HIV
Clin Trials. 2010;11:332-9.
|
|
|
 |
|

