|
|
|
Indian Pediatr 2015;52:
1041-1045 |
 |
Relationship between
Packed Red Blood Cell Transfusion and Severe Form of Necrotizing
Enterocolitis: A Case Control Study
|
|
*Parvesh M Garg, *Srikanth Ravisankar, Hui Bian,
*Scott Macgilvray And #Prem
S Shekhawat
From Departments of Biostatistics and *Pediatrics,
Brody School of Medicine at East Carolina University, Greenville, NC;
and #Department of Pediatrics, Division Neonatology,
MetroHealth Medical Center, Case Western Reserve University, Cleveland,
Ohio; USA.
Correspondence to: Dr Prem Shekhawat, Department of
Pediatrics, Division of Neonatology, MetroHealth Hospital, Case Western
Reserve University, 2500 MetroHealth Drive, Room R 249A, Cleveland, Ohio
44109, USA.
Email:
[email protected]
Received: March 13, 2015;
Initial review: May 01, 2015;
Accepted: October 08, 2015.
|
Objective: To determine if packed red blood cell transfusion is
associated with onset of necrotizing enterocolitis, and whether
withholding feed has any association with it.
Methods: Case records of 100 preterm neonates,
(<34 weeks gestation) who developed necrotizing enterocolitis and 99
random age-and gestation-matched controls were evaluated for any blood
transfusion 48 h before onset of necrotizing enterocolitis.
Results: During the study period 26% infants
received packed red blood cell transfusion within 48-hours prior to
onset of disease and 84% of these infants were not fed around the time
of transfusion. Infants who developed necrotizing enterocolitis after
transfusion were older, of lower gestational age, birth weight and more
likely to develop stage 3 disease. They had a lower hematocrit at birth
and before onset of disease and withholding feeds around transfusion did
not prevent necrotizing enterocolitis. Odds of mortality in these
infants was 2.83 (95% CI 0.97-8.9) and survivors had no significant
difference in incidence of periventricular leukomalacia and length of
hospital stay.
Conclusion: Blood Transfusion associated
necrotizing enterocolitis is a severe, mainly surgical form of disease.
Keywords: Blood transfusion, Feeding, Periventricular
leukomalacia, Prematurity.
|
|
Necrotizing enterocolitis (NEC) afflicts 6-10% of
very low birth weight (<1500 g) infants, and leads to higher morbidity,
mortality and increased length of hospital stay [1]. Several risk
factors such as intestinal immaturity, a genetic predisposition and
abnormal microbial colonization of the intestines predispose premature
infants to develop NEC [2]. Recently an association between receiving
packed red blood cell (PRBC) transfusions and the onset of NEC has been
observed [3-5]. Majority of very low birth weight (VLBW) infants will
require one or more PRBC transfusions during the course of their
neonatal intensive care unit (NICU) stay. It is unclear whether
withholding enteral feedings around the time of transfusion is effective
in reducing or preventing transfusion-associated NEC. We undertook this
study to observe association of PRBC transfusion and NEC at our
institution, and whether the practice of withholding feeds around the
time of PRBC transfusion is helpful in prevention of this type of NEC.
Methods
Our study was conducted in the Level 3b NICU at
Vidant Medical Center that serves as the regional referral center for 29
counties in Eastern North Carolina, caring for both inborn and outborn
infants with over 1000 admissions to the NICU annually. It is affiliated
teaching hospital for the Brody School of Medicine at East Carolina
University. This study was Health Insurance Portability and
Accountability Act (HIPPA) compliant and approved by the combined
Institutional Review Board of East Carolina University and Vidant
Medical Center.
Our institutional database was searched for all NICU
admissions the time period from January 1, 2002 through December 31,
2011 to identify infants with a gestational age at birth
£34 weeks who
developed stage 2a or greater NEC using modified Bell's Criteria [6].
Infants with known chromosomal anomalies, congenital heart disease, or a
diagnosis of an isolated spontaneous intestinal perforation were
excluded. Eligible matched controls were identified by matching 1 to 1
with each case patient for gestational age at birth (±10 days),
admission date (±4 weeks) and birth weight (±150g). The electronic
medical records for each of these cases and controls were then reviewed
and data elements collected for further analysis.
Maternal and infant characteristics historically
associated with an increased risk of developing NEC were recorded.
Maternal factors included the following: pregnancy induced hypertension,
chorioamnionitis, administration of antenatal steroids, preterm
premature rupture of membranes (PPROM), and prolonged rupture of
membranes (PROM). Infant data elements collected included; gestational
age at birth, birth weight, gender, mode of delivery, Apgar scores at 1
and 5 minutes, pre-transfusion hematocrit, corrected gestational age at
onset of NEC, relationship of NEC onset to a transfusion in the
preceding 48 hours, and severity of NEC along with any therapy provided.
Additional infant data elements included the presence of a
hemodynamically significant patent ductus arteriosus (PDA) and
treatment, if any, hypotension and therapy provided, type of feeding
(breast milk or formula feedings), administration of postnatal steroids
for prevention or treatment of chronic lung disease, and enteral feeding
status at the time of any transfusion. Data on outcome variables
included: diagnosis of bronchopulmonary dysplasia (BPD) as defined by
oxygen requirement at 36 weeks corrected gestational age,
periventricular leucomalacia (PVL) as defined by ultrasonography scan at
36 weeks corrected gestational age, retinopathy of prematurity (ROP),
length of stay, and death prior to hospital discharge. The decision to
provide a PRBC transfusion was at the discretion of attending physician.
Our hospital policy throughout the study period was that infants who
required a PRBC transfusion were transfused with PRBC that had been
stored for less than five days. We did not divide an adult unit of blood
into aliquots to limit donor exposure. All transfused PRBC units were
cytomegalovirus (CMV) negative, irradiated and leuko-reduced. Donor
blood was collected and stored in citrate phosphate dextrose (CPD)
solution.
Statistical methods: Statistical analyses were
performed with SPSS 20. Chi-square tests and Fisher's exact tests were
done to measure degree of association between categorical variables.
Analysis of variance (ANOVA) was used for comparisons involving
continuous variables between NEC and non-NEC. A simple logistic
regression was performed to calculate odds of having major outcomes
variables like mortality and PVL. Statistical significance was set at a
P <0.05.
Results
Over the ten year period from January 1, 2002 to
December 31, 2011, there were 9022 admissions to the NICU, and 131
neonates were identified as having had NEC. Thirty-one infants did not
meet the predetermined inclusion criteria leaving a total of 100
patients with NEC for evaluation (Fig. 1).
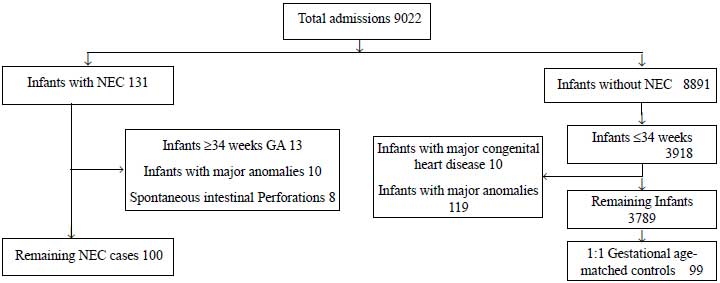 |
|
Fig. 1 Patient and control selection
flow chart with inclusion and exclusion criteria.
|
The overall incidence of NEC during the study period
was 1.45% of all admissions to the NICU, and 3.67% in infants
£34 weeks gestation.
The incidence of NEC did not vary significantly at our center during the
10 year study period. The NEC and matched control groups were similar in
gestational age at birth and most other demographic variables but
transfusion-associated NEC infants were significantly smaller in birth
weight compared to other infants with NEC and non NEC controls (P<0.05)
(Table I). There were more female and Hispanic subjects in
the transfusion-associated NEC group, and a larger proportion of them
had hemodynamically significant PDA which required surgical ligation (P<0.05).
A significantly greater number of mothers of transfusion-associated NEC
infants were diagnosed with pregnancy-induced hypertension as compared
to controls (Table I).
TABLE I Demographic Characteristics of NEC Infants and Controls
|
Transfusion-associated NEC (n=26)
|
Other NEC (n=73) |
Controls (n=99) |
|
*Gestational age (Wks)
|
27.3 (2.5) |
29.2 (3.0) |
28.7 (2.9) |
|
*Birth weight (g) |
992.8 (377.6) |
1287.7 (457.2) |
1260.7 (785) |
|
#Male infants |
5 (19.2) |
46 (63.0) |
55 (55.6) |
|
Race: White |
4 (15.4) |
19 (26.0) |
30 (30.3) |
|
Race: African American |
18 (69.2) |
51 (69.9) |
67 (67.7) |
|
Caesarian delivery
|
15 (57.7) |
35 (48.6) |
65 (65.7) |
|
Apgar score >6 at 5 min
|
11 (42.3) |
55 (75.3) |
63 (64.9) |
|
#PDA
|
15 (57.7) |
28 (38.4) |
46 (46.5) |
|
Feeds: Breast Milk |
10 (40) |
27 (39.1) |
39 (50.6) |
|
*Hematocrit at Birth
|
42.6 (6.9) |
47.7 (8.1) |
45.5 (7.7) |
|
Pregnancy induced hypertension
|
3 (11.5) |
12 (16.4) |
3 (3.0) |
|
NEC: Necrotizing enterocolitis; PDA:patent ductus arteriosus
Values in No. (%) or *mean (SD); #Significantly (P<0.05)
different between transfusion-associated and other NEC group. |
Infants with transfusion-associated NEC had a
significantly lower hematocrit at birth, and also a lower hematocrit
just before onset of NEC (Table II). Infants with
transfusion-associated NEC were of lower gestational age at birth, had
lower birth weight, and were more likely to have had a 5 minute Apgar
score of less than 6. Infants in the transfusion-associated group were
more likely to be of blood type B+ and less likely to be blood type A+.
The rates of premature rupture of membranes, maternal PIH and absent end
diastolic umbilical flow were similar between the two groups of NEC.
TABLE II Clinical Status at Onset of NEC and Outcomes in Two Groups
|
Transfusion-associated NEC (n=26) |
Other NEC (n=73) |
P value |
|
*Hematocrit at birth (%)
|
42.6 (6.9)
|
47.7 (8.1)
|
0.006 |
|
*Pre-transfusion hematocrit (%) |
27.4 (4.5)
|
34.5 (8.5)
|
<0.001
|
|
*Age at onset of NEC (d) |
28.4 (13)
|
19.0 (14.9)
|
0.005 |
|
Feeding withheld around transfusion |
22 (84.6) |
25 (36.8%) |
<0.001 |
|
Deaths |
8 (30.8) |
10 (13.7) |
0.08 |
|
ROP |
8/20 (40.0) |
21/54 (38.9) |
0.93 |
|
PVL |
4/24 (16.7) |
5/63 (7.9) |
0.25 |
|
*Length of stay (d) |
73.5 (48.6)
|
54.3 (44.6)
|
0.07 |
|
NEC: Necrotizing enterocolitis; ROP: Retinopathy of
prematurity; PVL: Perventricular leukomalacia; Values in No. (%)
or * mean (SD). |
Infants with transfusion-associated NEC were older at
the time when NEC developed, and had a higher rate of surgical (stage
3a+3b) NEC. They were also more likely to have had their feedings held
around the time of blood transfusion.
Discussion
Our study demonstrated an association between PRBC
transfusion and onset of NEC in about a quarter of our infants over the
10-year study period. These infants were smaller in their birth weight,
were born at lower gestational age, and were more likely to have had a
hemodynamically significant PDA. Infants in our study did not
demonstrate a protective effect of withholding feedings before blood
transfusion.
This main limitations of the study are: a
retrospective observational design; predominantly African-American
population, and a small sample size. Absence of a standard feeding
protocol or a policy on withholding feedings at the time of transfusion
during the study period precluded demonstration of a strong relationship
between PRBC transfusion and NEC.
Some recent studies have identified a similar
association between transfusion and transfusion-associated NEC [5,
7-15]. A meta-analysis of several of these studies concluded that there
is a strong association between PRBC transfusion and odds of developing
NEC [16]. A more recent meta-analysis has been more cautious in reaching
the same conclusion [17]. On the other hand, two recent reports did not
find PRBC transfusion as a risk factor for NEC [18,19]. Some
investigators have reported a significant decrease in the overall NEC
rate following the institution of a policy of withholding enteral
feedings during and after PRBC transfusion [20]. However, in our study,
84.6% of transfusion-associated NEC cases had their enteral feedings
withheld during the time of transfusion as well as for several hours
afterwards, suggesting that withholding enteral feedings around the time
of a transfusion may not prevent NEC.
In conclusion, our study suggests a temporal
association of a PRBC transfusion with the development of NEC that has
also been reported by others. Our data also suggest that infants with
transfusion-associated NEC represent a subset that is more likely to be
seen in smaller VLBW infants. Withholding enteral feedings around the
time of a PRBC transfusion also does not seem to be an effective
strategy to decrease the occurrence of transfusion-associated NEC.
Contributors: PS, PG: conceptualized this study;
PG, PS, SR, SM: extracted data and all authors analyzed data and
contributed in writing and approval of the manuscript. Funding:
None; Competing interests: None stated.
|
What is Already Known?
• Onset of Necrotizing enterocolitis is
preceded by blood transfusion in some cases and it leads to a
severe form of disease with high morbidity and mortality.
What This Study Adds?
• Blood transfusion-associated Necrotizing
enterocolitis seems to be a severe form of disease and
withholding feedings around the time of transfusion does not
seem to prevent this entity.
|
References
1. Neu J, Walker WA. Necrotizing enterocolitis. N
Engl J Med. 2011;364:255-64.
2. Neu J, Douglas-Escobar M, Lopez M. Microbes and
the developing gastrointestinal tract. Nutr Clin Pract. 2007;22:174-82.
3. Christensen RD, Lambert DK, Henry E, Wiedmeier SE,
Snow GL, Baer VL, et al. Is "transfusion-associated necrotizing
enterocolitis" an authentic pathogenic entity? Transfusion.
2010;50:1106-12.
4. Bishara N, Ohls RK. Current controversies in the
management of the anemia of prematurity. Semin Perinatol. 2009;33:29-34.
6. Josephson CD, Wesolowski A, Bao G, Sola-Visner MC,
Dudell G, Castillejo MI, et al. Do red cell transfusions increase
the risk of necrotizing enterocolitis in premature infants? J Pediatr.
2010;157:972-8 e1-3.
7. Walsh MC, Kliegman RM. Necrotizing enterocolitis:
treatment based on staging criteria. Pediatr Clin North Am.
1986;33:179-201.
8. Mally P, Golombek SG, Mishra R, Nigam S, Mohandas
K, Depalhma H, et al. Association of necrotizing enterocolitis
with elective packed red blood cell transfusions in stable, growing,
premature neonates. Am J Perinatol. 2006;23:451-8.
9. Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La
Gamma EF. Transfusion-related acute gut injury: necrotizing
enterocolitis in very low birth weight neonates after packed red blood
cell transfusion. J Pediatr. 2010;158:403-9.
10. Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A,
Locke RG. Increased odds of necrotizing enterocolitis after transfusion
of red blood cells in premature infants. Pediatrics. 2011;127:635-41.
11. Singh R, Visintainer PF, Frantz ID, Shah BL,
Meyer KM, Favila SA, et al. Association of necrotizing
enterocolitis with anemia and packed red blood cell transfusions in
preterm infants. J Perinatol. 2011;31:176-82.
12. Amin SC, Remon JI, Subbarao GC, Maheshwari A.
Association between red cell transfusions and necrotizing enterocolitis.
J Matern Fetal Neonatal Med. 2012;25(Suppl 5):85-9.
13. Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS.
Transfusion-associated necrotising enterocolitis in neonates. Arch Dis
Child Fetal Neonatal Ed. 2012; 2:445-567.
14. Bak SY, Lee S, Park JH, Park KH, Jeon JH.
Analysis of the association between necrotizing enterocolitis and
transfusion of red blood cell in very low birth weight preterm infants.
Korean J Pediatr. 2013;56:112-5.
15. Wan-Huen P, Bateman D, Shapiro DM, Parravicini E.
Packed red blood cell transfusion is an independent risk factor for
necrotizing enterocolitis in premature infants. J Perinatol.
2013;33:786-90.
16. Gephart SM, Spitzer AR, Effken JA, Dodd E,
Halpern M, McGrath JM. Discrimination of GutCheck (NEC): a clinical risk
index for necrotizing enterocolitis. J Perinatol. 2014;34:468-75.
17. Mohamed A, Shah PS. Transfusion associated
necrotizing enterocolitis: a meta-analysis of observational data.
Pediatrics. 2012;129:529-40.
18. Kirpalani H, Zupancic JA. Do transfusions cause
necrotizing enterocolitis? The complementary role of randomized trials
and observational studies. Semin Perinatol. 2012;36:269-76.
19. Sharma R, Kraemer DF, Torrazza RM, Mai V, Neu J,
Shuster JJ, et al. Packed red blood cell transfusion is not
associated with increased risk of necrotizing enterocolitis in premature
infants. J Perinatol. 2014;34:858-62.
20. Wallenstein MB, Arain YH, Birnie KL, Andrews J,
Palma JP, Benitz WE, et al. Red blood cell transfusion is not
associated with necrotizing enterocolitis: a review of consecutive
transfusions in a tertiary neonatal intensive care unit. J Pediatr.
2014;165:678-82.
21. Viswanathan S, McNelis K, Super D, Einstadter D,
Groh-Wargo S, Collin M. A Standardized slow enteral feeding protocol and
the incidence of necrotizing enterocolitis in extremely low birth weight
infants. J Parenter Enteral Nutr. 2015;39:644-54.
|
|
|
 |
|

