|
|
|
Indian Pediatr 2012;49: 979-982
|
 |
Effect of Infliximab ‘Top-down’ Therapy on
Weight Gain in Pediatric Crohn’s Disease
|
|
Mi Jin Kim,
*Woo Yong Lee,
Kyong Eun Choi AND Yon Ho Choe
From the Department of Pediatrics, and *Department of
Surgery, Sungkyunkwan University School of Medicine, Samsung Medical
Center, , Seoul, Korea.
Correspondence to: Dr Yon Ho Choe, Department of
Pediatrics, Samsung Medical Center, Sungkyunkwan University School of
Medicine, 50 Irwon-dong, Gangnam-gu, Seoul 135-710, Korea.
Email: [email protected]
Received: November 04, 2011;
Initial review: January 10, 2012;
Accepted: April 23, 2012.
Published online: June 10, 2012.
PII: S097475591100913-2
|
This retrospective-medical-record review was conducted to evaluate
effect of infliximab therapy, particularly with a ‘top-down’ strategy,
on the nutritional parameters of children with Crohn’s disease (CD). 42
patients who were diagnosed with Crohn’s disease at the Pediatric
Gastroenterology center of a tertiary care teaching hospital and
achieved remission at two months and one year after beginning of
treatment were divided into four subgroups according to the treatment
regimen; ‘azathioprine’ group (n = 11), ‘steroid’ group (n
= 11), infliximab ‘top-down’ group (n = 11) and ‘step-up’ group (n
= 9). Weight, height, and serum albumin were measured at diagnosis, and
then at two months and one year after the initiation of treatment. At 2
months, the Z–score increment for weight was highest in the
‘steroid’ group, followed by the ‘top-down’, ‘step-up’, and ‘azathioprine’
groups. At one year, the Z–score increment was highest in
‘top-down’ group, followed by ‘steroid’, ‘azathioprine’, and ‘step-up’
group. There were no significant differences between the four groups in
Z–score increment for height and serum albumin during the study
period. The ‘top-down’ infliximab treatment resulted in superior outcome
for weight gain, compared to the ‘step-up’ therapy and other treatment
regimens.
Key words: Children, Crohn’s disease, Infliximab, Top-down
strategy, Weight gain.
|
|
Crohn’s disease (CD) is characterized by
chronic inflammation involving any portion of the
gastrointestinal tract, and the inflammation contributes to
growth retardation affecting both height and weight [1]. Current
practice guidelines recommend that most patients with active
disease should initially be treated with corticosteroids [2-4].
Although this approach is usually effective for symptom control,
many patients become either resistant or dependent on these
drugs [5] or suffer short-and long-term adverse effects. Over
the last decade, infliximab, a monoclonal immunoglobulin G1
chimeric antibody directed against tumor necrosis factor-a,
has become another therapeutic option for the induction and
maintenance of remission in children with severe CD. The
efficacy of infliximab suggests that, rather than a progressive
course of treatment, early intense (‘top-down’ strategy)
induction may reduce complications associated with conventional
treatment and improve quality of life.
The purpose of this study was to perform a
comparative evaluation of the effects of infliximab therapy with
other treatment modalities, on nutritional parameters for
pediatric patients with CD.
Methods
Among pediatric patients who were diagnosed
with CD in accordance with the European Society for Pediatric
Gastroenterology, Hepatology and Nutrition - Porto criteria [6]
at the Samsung Medical Center between March 2001 and March 2010,
we enrolled 42 age-matched patients who achieved remission at
two months and one year after initiation of treatment (Fig.
1). We had a protocol for nutritional evaluation including
height, body weight and serum albumin level and applied it in
all CD patients. A retrospective chart analysis was conducted by
physician notes, laboratory studies, radiology reports,
endoscopy records, and histology reports. Patients with severe
malnutrition affecting growth and development who required
parenteral or enteral nutrition were excluded. This study was
approved by our institutional review board.
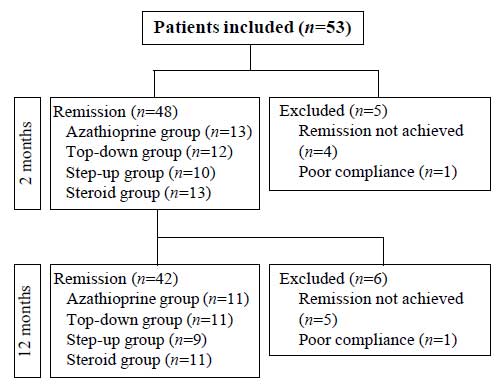 |
|
Fig.1 Diagrammatic flow of the
study.
|
The patients (n=42) were divided into
four subgroups according to the treatment regimen. Eleven
patients (‘azathioprine’ group) were treated with azathioprine
and 11 patients (‘steroid’ group) were treated with oral
prednisolone for induction and 11 patients (‘top-down’ group)
were given infliximab from the beginning of treatment. Nine
patients (‘step-up’ group) who had been refractory to
conventional therapy were treated with infliximab (Table
I). Simultaneously all patients were receiving mesalamine (Pentasa,
50-80 mg/kg per day).
TABLE I Baseline Parameters of the Study Population
|
Aza (n=11) |
TD (n=11) |
SU (n=9) |
Steroid (n=11) |
|
Gender, male/female |
8/3 |
8/3 |
6/3 |
8/3 |
|
Age (y)
|
15 (11-18) |
14 (12-18)
|
14 (10-16) |
14 (11-16) |
|
PCDAI score at diagnosis*
|
15.0 (5.0-37.5) |
37.5 (17.5-55.0) |
40.0 (17.5-47.5) |
27.5 (12.5-42.5) |
|
at 2 mo
|
5.0 (0.0-10.0) |
2.5 (0.0-12.5) |
5.0 (0.0-15.0) |
2.5 (0.0-10.0) |
|
at 1 y after beginning treatment |
5.0 (0.0-20.0) |
0.0 (0.0-10.0) |
5.0 (0.0-15.0) |
0.0(0.0-20.0) |
|
All values except gender are median
(range); * P=0.001; PCDAI scores after beginning of
treatment; PCDAI, Pediatric Crohn’s Disease Activity
Index; Aza, azathioprine treatment group; TD, infliximab
‘top-down’ treatment group; SU, infliximab ‘step-up’
treatment group; Steroid, steroid treatment group.
|
In the ‘steroid’ and ‘step-up’ group, oral
corticosteroids (prednisolone, 1-2 mg/kg per day) were used for
induction therapy. Azathioprine (Imuran, 2-3 mg/kg per day) was
provided for maintenance therapy as the conventional treatment.
In the ‘step-up’ and ‘top-down’ group, infliximab (5 mg/kg) was
administered by intravenous infusion at weeks zero, two and six,
in combination with daily azathioprine, and this course was
repeated every eight weeks for ten months thereafter. The group
treated with ‘top-down’ infliximab had not been treated
previously with other medications such as corticosteroids or
other immunomodulators. All patients were followed for at least
12 months.
PCDAI score is calculated from 11 variables
and total score can range from 0 to 100 with higher score
indicating greater disease activity. PCDAI scores were measured
at diagnosis, and then at two and 12 months after the beginning
of treatment. We defined disease remission as a PCDAI score of
less than 10 points and relapse as a score greater than 10
points [7,8]. Moderate to severe disease was defined as having a
score greater than 30 points. Azathioprine was used for patients
with a mild to moderate PCDAI score, and infliximab was used for
patients with a moderate to severe score.
Weight and height were measured at diagnosis,
and then at two months and one year after the beginning of
treatment, except for the ‘step-up’ group. In the ‘step-up’
group, weight and height were measured at the beginning of
infliximab treatment, and then at two months and one year later.
The Z score (standard deviation
scores) was used to evaluate and compare anthropometric
measurements for CD children of various age and gender. A growth
chart for Korean children was used as a reference for body
composition.
Statistical analyses were performed using
Mann-Whitney U-test for unpaired samples and Wilcoxon
signed-rank test for paired samples. Analyses were performed
using Kruskall-Wallis test with Bonferroni’s correction and
Behrens-Fisher method for nonparametric multiple comparisons
(SPSS, Chicago, IL, USA). A value of P < 0.05 was
regarded as statistically significant.
Results
Comparison of baseline parameters in the 4
groups are shown in Table I. At two months
following the start of treatment, in the ‘top-down’ group, the
increment in Z-score for weight was superior to those in
the ‘azathioprine’ and ‘step-up’ group (P=0.010 and P=0.036,
respectively). In the ‘steroid’ group, the increment in Z
score was also superior to those in the ‘azathioprine’ and
‘step-up’ group (P=0.001 and P=0.002,
respectively). At one year, in the ‘top-down’ group, the
increment in Z score for weight was superior to those for
the ‘azathioprine’ and ‘step-up’ groups (P=0.009 and P=0.001,
respectively). In the ‘steroid’ group, the increment in Z
score was superior to those in the ‘step-up’ group (P=
0.003). At one year, there were no significant differences
between the four groups in Z score increment for height
and serum albumin at diagnosis, or at two months or one year
after the beginning of treatment (Table II).
TABLE II Z score Increments and Albumin Levels in The Study Population
|
Median Z score increments (range) |
Median albumin levels (g/dL) (range) |
|
Weight |
Height |
Baseline |
2 months |
1 year |
|
Baseline |
2 months* |
1 year# |
Baseline |
2 months |
1 year |
|
|
|
|
Aza |
-0.57 |
0.15 |
0.43 |
-0.25 |
0.28 |
0.56 |
3.8 |
4.1 |
4.3 |
|
(-1.4-2.1) |
(-0.7-0.7) |
(0.0 – 1.5) |
(-2.0 -1.6) |
(-0.1-0.6) |
(0.0-1.0) |
(1.9 -5.1) |
(3.3-4.6) |
(3.4-4.8) |
|
TD |
-0.72 |
0.42 |
0.97 |
-0.48 |
0.18 |
0.67 |
3.5 |
4.4 |
4.4 |
|
(-2.5-2.0) |
(-0.1-0.6) |
(0.0 – 1.5) |
(-2.0 -1.0) |
(-0.2-0.5) |
(0.0 -1.5) |
(2.6 -4.6) |
(3.0 -4.9) |
(3.9-5.0) |
|
SU |
-0.44 |
0.11 |
0.37 |
-0.08 |
0.21 |
0.44 |
3.6 |
3.9 |
4.0 |
|
(-1.9-2.2) |
(-0.1-0.5) |
(-0.9 – 0.8) |
(-1.5-2.3) |
(0.0 -0.4) |
(-0.5-1.4) |
(2.4-4.5) |
(2.9-4.6) |
(3.5-4.6) |
|
Steroid |
-0.53 |
0.66 |
0.73 |
-0.18 |
0.34 |
0.54 |
3.2 |
4.0 |
4.1 |
|
(-1.6-2.0) |
(0.1-0.9) |
(0.1-.5) |
(-1.8-1.2) |
(0.0 -0.9) |
(0.2-1.6) |
(2.5-4.2) |
(3.7-4.5) |
(3.6 -4.7) |
|
Aza, azathioprine treatment group; TD, infliximab
‘top-down’ treatment group; SU, infliximab ‘step-up’
treatment group; Steroid, steroid treatment group;* |
Discussion
One of the critical aims of management in
pediatric CD is growth. Malnutrition is a major treatable cause
of growth failure in inflammatory bowel disease, with weight
loss being present in up to 80% of patients with CD at
presentation [9]. Thus, it is essential to evaluate the outcomes
of specific therapies in terms of their benefit on growth. To
our knowledge, this is the first study to show differences in
the improvement in body weight according to treatment regimens.
Weight, height, and serum albumin level increased during the
treatment period. There was only a significant difference
between treatment groups for weight at a year after treatment.
For height, the one-year follow-up did not seem to be enough to
evaluate linear growth. Serum albumin was restored to a normal
level at 2 months in all groups who achieved remission.
Growth failure mostly appears to be due to
disease activity, with smaller nutritional and iatrogenic
components [10]. The significant difference in weight gain
between ‘top-down’ and ‘azathioprine’ or ‘step-up’ group is most
likely due to the fact that patients of ‘top-down’ group were
malnourished at baseline because of more severe disease.
Infliximab in ‘top-down’ group led to significant improvement in
disease activity and therefore there was marked increase of
weight in this group.
At 2 months, ‘steroid’ group showed the
highest z-score increment of weight, which is because steroids
generally induce short-term weight gain due to increased
appetite. At one year, the ‘top-down’ group showed the highest
Z score increment. We assume that the ‘step-up’ group
placed last at one year because the ‘azathioprine’ group
initially included patients with relatively mild to moderate
PCDAI score.
The current study was limited in that it was
a single-center retrospective study with a small number of
patients, and the follow-up period was only one year. We tried
to include all patients fulfilling the inclusion criteria;
however, selection bias did not disappear completely.
In summary, we found that ‘top-down’
infliximab treatment resulted in a superior outcome for weight
gain, compared to ‘step-up’ therapy and other treatment
regimens. In clinical practice, growth should be carefully
considered as an important criterion for management of CD
children and an important marker of therapeutic efficiency.
Future studies addressing long-term follow-up are needed to
determine the efficacy of infliximab treatment.
Contributors: MJK and YHC:
conceived and designed the study and revised the manuscript for
important intellectual content; YHC: will act as guarantor of
the study; WYL and KEC: analyzed the data and helped in
manuscript writing. The final manuscript was approved by all
authors.
Funding: Grant of the Korea Healthcare
technology R&D Project, Ministry for Health & Welfare Affairs,
Republic of Korea (A092255), and Samsung Biomedical Research
Institute grant, no. SBRI C-A6-229-3; Competing interests:
None stated.
|
What This Study Adds?
•
‘Top-down’ infliximab treatment resulted in superior
outcome for weight gain, compared to the ‘step-up’
therapy and other treatment regimens.
|
References
1. Simon D. Inflammation and growth. J
Pediatr Gastroenterol Nutr. 2010;51 (Suppl 3):S133-4.
2. Van Limbergen J, Russell RK, Drummond HE,
Aldhous MC, Round NK, Nimmo ER, et al. Definition of
phenotypic characteristics of childhood-onset inflammatory bowel
disease. Gastroenterology. 2008;135:1114-22.
3. Malchow H, Ewe K, Brandes JW, Goebell H,
Ehms H, Sommer H, et al. European Cooperative Crohn’s
Disease Study (ECCDS): results of drug treatment.
Gastroenterology. 1984;86:249-66.
4. Summers RW, Switz DM, Sessions JT, Jr.,
Becktel JM, Best WR, Kern F, Jr., et al. National
Cooperative Crohn’s Disease Study: results of drug treatment.
Gastroenterology. 1979;77:847-69.
5. Munkholm P, Langholz E, Davidsen M, Binder
V. Frequency of glucocorticoid resistance and dependency in
Crohn’s disease. Gut. 1994;35:360-2.
6. IBD Working Group of the European Society
for Paediatric Gastroenterology HaN. Inflammatory bowel disease
in children and adolescents: recommendations for diagnosis—the
Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1-7.
7. Hyams J, Markowitz J, Otley A, Rosh J,
Mack D, Bousvaros A, et al. Evaluation of the pediatric
crohn disease activity index: a prospective multicenter
experience. J Pediatr Gastroenterol Nutr. 2005;41:416-21.
8. Otley A, Loonen H, Parekh N, Corey M,
Sherman PM, Griffiths AM. Assessing activity of pediatric
Crohn’s disease: which index to use? Gastroenterology.
1999;116:527-31.
9. Thayu M, Denson LA, Shults J, Zemel BS,
Burnham JM, Baldassano RN, et al. Determinants of changes
in linear growth and body composition in incident pediatric
Crohn’s disease. Gastroenterology. 2010;139:430-8.
10. Motil KJ, Grand RJ, Davis-Kraft L, Ferlic
LL, Smith EO. Growth failure in children with inflammatory bowel
disease: a prospective study. Gastroenterology. 1993;105:681-91.
|
|
|
 |
|

