|
|
|
Indian Pediatr 2021;58:749-752 |
 |
Long term Immunogenicity of Single Dose of
Live Attenuated Hepatitis A Vaccine in Indian Children - Results
of 15-Year Follow-up
|
|
Sheila Bhave1, Amita
Sapru1,
Ashish Bavdekar1,
Rishi Jain2,
Khokan Debnath,2
Vaibhavi Kapatkar2
From 1Department of Pediatrics, KEM Hospital Research Centre, Pune
and 2Department of Medical Affairs, Wockhardt Limited, Mumbai;
Maharashtra.
Correspondence to: Dr Sheila Bhave, Consultant in Pediatric Research,
Department of Pediatrics, KEM Hospital Research Centre, Rasta Peth, Pune
411 011, India.
Email: [email protected]
Received: July 07, 2020;
Initial review: August 28, 2020;
Accepted: February 12, 2021
Published online: February 19, 2021;
PII: S097475591600293
|
Objectives: To measure anti-HAV antibodies 15
years after a single dose of live attenuated hepatitis A vaccine in
Indian children. Methods: Of the 143 children vaccinated in 2004,
109 were evaluated in 2019, clinically and for anti-HAV antibodies.
These children have been assessed clinically every year, and for
anti-HAV antibodies in 2004, 2007, 2010 and 2014. Results: Of the
109 children who came for the present assessment, 11 had received
additional doses of hepatitis A vaccine in 2004/2007 because of low
anti-HAV titre (<20 mIU/mL). In the remaining 98 children, 94 (96%) had
seroprotective levels with a geometric mean titre of 79.6 mIU/mL.
Seroprotection rate in all 109 children was 86.2%. Conclusions:
Single dose of live attenuated hepatitis A vaccine in Indian children
demonstrated robust immunogenicity at 15 years post vaccination.
Keywords: Hepatitis A vaccine, Anti-HAV anti bodies, Immune
memory, Safety.
|
|
L
ive attenuated
hepatitis A vaccine (H2 strain)
has a long history of development and research
(in China) for nearly three decades [1,2].
Promising immunogenicity, safety and protection have been
reported, using a single dose of the vaccine [3]. In India, a
live vaccine was licensed in 2005 and has been used extensively
since then. Immunogenicity studies of the single dose regimen
in India have matched the Chinese reports [3,4]. Both, World
Health Organization (WHO) and Indian Academy of Pediatrics (IAP)
have endorsed the single dose schedule of live hepatitis A
vaccine in the routine immunization of children aged one year or
above [5,6].
In this study, we report the anti-HAV
antibodies at 15 years from the first Indian study of single
dose live HAV vaccine in children. Immunogenicity data from the
same cohort at 2 months, 30 months and 10 years
post-immunization has previously been reported [7-9].
METHODS
The study began in 2004 wherein 143 children
were given a single dose of H2 strain of a live attenuated
hepatitis A vaccine (Biovac-A, Wockhardt Ltd) and assessed for
anti-HAV antibodies 2-months post vaccination [7]. These
children were then called for follow-up every year, for clinical
assessment and to record history of hepatitis, if any. They were
assessed serially for anti-HAV antibodies in 2007, 2010 and 2014
[8,9]. Subjects with low anti-HAV antibody titres (<20 mIU/mL)
were given additional doses of vaccine viz., in 2004 they were
given two doses of the then licensed HAV vaccine (Havrix Jr GSK
Biologicals) and in 2007 they received an additional dose of
Biovac-A vaccine. No further vaccines were given in 2010 or
2014.
Contact details of the cohort were maintained
by medical social workers. At the yearly visits,
participants/parents were asked for history of hepatitis like
illness (fever, anorexia, nausea, vomiting and jaundice).
Clinical examination included noting for hepatomegaly or
splenomegaly, if any. Parents were reminded to report complaints
of hepatitis like illness immediately. No diary was given to
participants for recording signs and symptoms. In the present
study (2019) too, these children were clinically assessed for
evidence of hepatitis (if any) and their anti-HAV antibodies
were measured.
After routine clinical assessments, blood
samples were collected and sent for total and IgM anti-HAV
antibody analysis (Cobas anti-HAV electro-chemilumi-nescence
immunoassay, ECLIA, Roche Diagnostics Deutschland GmbH) to an
independent accredited laboratory (SRL Diagnostics).
Seroprotection rate was defined as proportion of subjects with
total anti-HAV antibody level
³20 mIU/mL.
Geometric mean titre (GMT) for anti-HAV antibodies was
calculated as per standard method. Data was entered in
predesigned paper case report forms (CRFs). All study documents
were maintained in a dedicated study cupboard with restricted
access. Data analysis was done using Stata 13.1 (StataCorp). All
analysis was done using two-sided tests at alpha 0.05 (95%
confidence level).
During the study of 15 years, institutional
ethics committee approval and informed consent were obtained
three times: Initial study (2004), second phase of study
(2007-2015), and third phase of study (2016-2019). During the
study, whenever a participant attained the age of 18 years,
informed consent was obtained from him/her for continuing in the
study.
RESULTS
Of the original 143 children who received a
single dose of live attenuated hepatitis A vaccine in 2004, 109
subjects (72 males) came for the fifteen-year follow-up
assess-ment in 2019. Mean age was 19.7 years (range 16-26.8
years). The number of subjects who came for follow-up since
vaccination is as follows: 2.5 years (n=131), 6 years (n=126),
10 years (n=121) and 15 years (n=109). None of the
parents/children retracted consent in writing. Clinical
examination of the participants did not reveal any abnormal
findings, and none gave any history of hepatitis like illness in
the past.
Of the 109 children who came for the present
follow-up, 4 had received two doses of licensed inactivated HAV
vaccine in 2004, and 7 others had received a second dose of live
HAV vaccine in 2007, as their total anti-HAV antibody levels had
dropped to <20mIU/mL.
Of the remaining 98 children, 4 had low
anti-HAV titres (<20 mIU/mL) giving a seroprotection rate of
95.9%. If the 11 children who were given additional doses of HAV
vaccine are also included, the seroprotec-tion rate in all 109
children was 86.2%. The comparison of sero-protection rate in
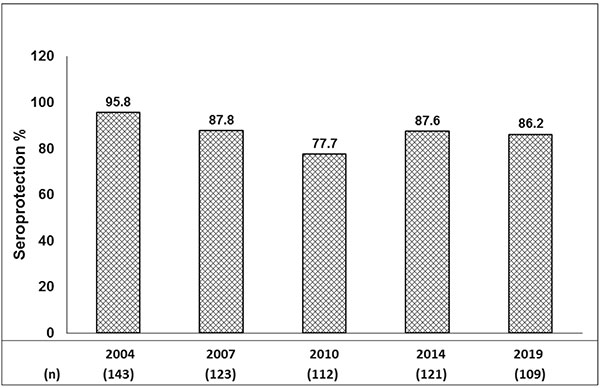
2019 with previous assessment years is shown in Fig. 1.
 |
|
Fig. 1 Serial seroprotection
rates over 15 years (%).
|
All children were found to be negative for
anti-HAV IgM. The total anti-HAV geometric mean titre (GMT) in
‘seroprotected children’ who received single dose of live
attenuated vaccine (n=94) is 79.6 mIU/mL (95% CI
69.2-91.56). The comparison of GMT value at 15 years with
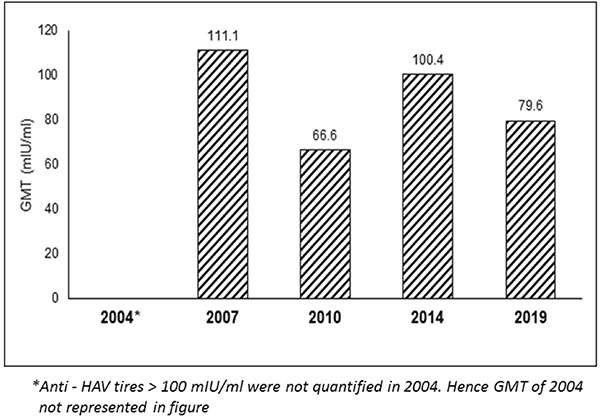
previous assessment years is shown in Fig. 2.
 |
|
Fig. 2 Serum anti-HAV antibody
GMTs over 15 years.
|
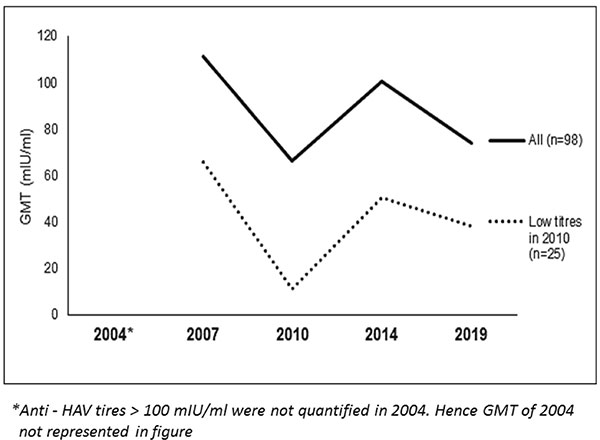
In 2010, there were 25 children with anti-HAV
titres <20mIU/mL. They were not given any additional dose /
doses of live/inactivated HAV vaccine. The serial anti-HAV GMTs
of these 25 children as compared to all 98 with single dose of
live HAV vaccine is shown in Fig. 3. In 2014 and 2019, 23
of these 25 regained seroprotective levels. In 2019, the
anti-HAV antibody titre of two other children (who had
seroprotective levels earlier) are now < 20 mIU/mL.
 |
|
Fig. 3 Serum anti-HAV antibody
GMTs of children with low titers in 2010.
|
Children who received two additional doses of
an inactivated HAV vaccine in 2004 (n=4), or an
additional dose of live HAV vaccine in 2007 (n=7) have
continued to show seroprotective levels since additional
vaccination.
DISCUSSION
This 15-year follow-up of a cohort of
children vaccinated with a single dose of live attenuated HAV
vaccine shows a seroprotective rate of 86.2% with anti HAV GMT
value of 79.6 mIU/mL. The serial seroprotection rates of the
cohort are 95.8% at 2 months, 87.8% at 30 months, 77.7% at 6
years, 87.6% at 10 years and 86.2% at 15 years. This evaluation
at 15 years confirms the robust long-term immunogenicity of a
single dose of live HAV vaccine and compares well with other
Indian and Chinese studies [3,10-12]. The comparable long term
Chinese seroprotection data (Zhuang, et al.) at 15 years is
81.3% (GMT 128 mIU/mL) [2,3]. The other Indian long term
multicentric study reported an immunogenicity of 97.3% at 5
years with GMT of 127.1mIU/mL [11].
In our serial evaluations since 2004, we used
two types of immunoassay test kits: Axsym HAVB ELISA (Abbott
Labs), (2004, 2007 and 2010). These kits were not available in
India in 2014. Hence, we used COBAS kits based on ECLIA
technology (Roche Diagnostics) in 2014 and 2019.The higher
antibody titres of 2014 and 2019 could be due to differences in
kits as ECLIA based reports are known to be of a higher
sensitivity as compared to ELISA [13]. Alternatively, higher
titres could be due to ‘booster like’ response to exposure to
naturally occurring antigens of HAV in the community [14]. As
the child grows from a teenager to adulthood, the frequency of
consuming food and water outside the home increases, thereby
increasing exposure to hepatitis A.
Another limitation of our study was cohort
contamination with additional doses of hepatitis A vaccine [9].
In 2004, 6 children were given two doses of inactivated HAV
vaccine and in the next evaluation at 30 months, 9 others were
given a second dose of the live HAV vaccine. At the 6 yrs
follow-up in 2010, 25 children were found to be seronegative.
These 25 children were not given any additional doses of
vaccine. Interestingly, 23 of these 25 were in the seroprotected
range in the present evaluation, further implying the
probability of an anamnestic response to natural boosters. Chen,
et al. [15] have recently demonstrated that anamnestic responses
via memory B and memory T cells may provide long term protection
after a single dose of live Hepatitis A vaccine, despite low
levels of anti-HAV antibodies.
In conclusion, 15-year follow-up after a
single dose of live hepatitis A vaccine (H2 strain) demonstrated
robust immunogenicity in Indian children. The continued safety
and immunogenicity profile of the vaccine reiterates its value
in primary immunization of Indian children. As a policy
decision, the single dose schedule cuts costs while providing
definitive long-term protection.
Acknowledgements: Dr Sonali Shah for data
management and follow-up; Dr Deepak Langade (Clinsearch
Healthcare Solutions Pvt Ltd) for statistical inputs; Dr Archana
Karadkhele (Wockhardt Ltd) & Dr Pramit Sonone (Wockhardt Ltd)
for valuable inputs.
Ethics clearance: KEM
Hospital Research Centre Ethics Committee; No. KEMHRC/LFG/
EC/778 dated June 6, 2016.
Contributors: SB, AS, AB: designed
the study, recruited patients, analyzed results and wrote the
manuscript; RJ, KD, VK: provided technical help needed for the
study.
Funding: Wockhardt Ltd. Recipient
of funds is KEM Hospital Research Centre, Pune.
Competing interests: SB, AS and
AB: received investigator fee for conduct of the study; RJ, KD
and VK are paid employees of Wockhardt Ltd.
|
WHAT THIS STUDY ADDS?
• A single dose of live attenuated
hepatitis A vaccine shows robust immunogenicity with
seroprotection level of 86.2% at 15 years after
vaccination in Indian children
|
REFERENCES
1. Mao JS, Dong DX, Zhang HY, et al.
Primary study of attenuated live hepatitis A vaccine (H2
strain) in humans. J Infect Dis. 1989;159:621-24.
2. Zhuang FC, Qian W, et al. Persistent
efficacy of live attenuated hepatitis A vaccine (H2-strain)
after a mass vaccination program. Chin Med J (Engl). 2005;118:1851-856.
3. Zhuang FC, Mao ZA, Jiang LM, et al.
Long term immunogenicity and effectiveness of live
attenuated hepatitis A vaccine (H2-strain) – A study on the
result of 15 years’ follow up. Zhonghua Liu Xing Bing Xue Za
Zhi. 2010;31:1332-335.
4. Shah N, Faridi MMA, Mitra M, et al.
Review of long term immunogenicity and tolerability of live
hepatitis A vaccine. Hum Vaccin Immunother. 2020 Apr 3;1-6
5. WHO position paper on hepatitis A
vaccines – June, 2012. Weekly Epidemiological Record.
2012;87:261-76.
6. Pemde H. Hepatitis A vaccines. In:
Advisory Committee on Vaccines and Immunization
Practices, Indian Academy of Pediatrics. IAP Guidebook on
Immunization 2018–2019. 3rd edition. Jaypee Brothers Medical
Publishers; 2020. p. 265-78.
7. Bhave S, Bavdekar A, Madan Z, et al.
Evaluation of immunogenicity and tolerability of a live
attenuated hepatitis A vaccine in Indian children. Indian
Pediatr. 2006;43:983-7
8. Bhave S, Sapru A, Bavdekar A,
Bawangade S, Pandit A. Immunogenicity of single dose live
attenuated hepatitis A vaccine. Indian Pediatr.
2011;48:135-37.
9. Bhave S, Sapru A, Bavdekar A, Kapatkar
V, Mane A. Long-term immunogenicity of single dose of live
attenuated hepatitis A vaccine in Indian children. Indian
Pediatr. 2015; 52:687-90.
10. Faridi MMA, Shah N, Ghosh TK, et al.
Immunogenicity and safety of live attenuated hepatitis A
vaccine: A multicentric study. Indian Pediatr.
2009;46:29-34.
11. Mitra M, Shah N, Faridi MMA, et al.
Long term follow-up study to evaluate immunogenicity and
safety of a single dose of live attenuated hepatitis A
vaccine in children. Hum Vaccin Immunother.
2015;11:1147-152.
12. Wang XY, Xu ZY, Ma JC, et al. Long
term immunogenicity after single and booster dose of a live
attenuated hepatitis A vaccine: Results from 8-year
follow-up. Vaccine. 2007; 25:446-49.
13. Zhang QY, Chen H, Lin Z, Lin JM.
Comparison of chemiluminescence enzyme immunoassay based on
magnetic microparticles with traditional colorimetric ELISA
for the detection of serum
a-fetoprotein.
J Pharm Analysis. 2012;2:130-35.
14. Arankalle V, Mitra M, Bhave S, et al.
Changing epidemiology of hepatitis A virus in Indian
children. Vaccine: Development and Therapy. 2014;4:7-13.
15. Chen Y, Zhou C-L, Zhang X-J, et al. Immune memory at
17-years of follow up of a single dose of live attenuated
hepatitis A vaccine. Vaccine. 2018;36:114-21.
|
|
|
 |
|

