|
|
|
Indian Pediatr 2021;58: 718-722 |
 |
Clinical Profile and Short-Term Outcome of
Children With SARS-CoV-2 Related Multisystem Inflammatory
Syndrome (MIS-C) Treated With Pulse Methylprednisolone
|
Sheeja Sugunan, S Bindusha, S Geetha, HR Niyas, A
Santhosh Kumar
From Department of Pediatrics, SAT Hospital, Government
Medical College, Thiruvananthapuram, Kerala.
Correspondence to: Dr Sheeja Sugunan, Associate
Professor, Department of Pediatrics, SAT hospital,
Government Medical College Thiruvananthapuram, Kerala.
Email:
[email protected]
Received: February 07, 2021;
Initial review: February 25, 2021;
Accepted: April 19, 2021.
Published online:
April 20, 2021;
PII: S097475591600319
|
Objective: To study the clinical
profile and outcome of children with MIS-C treated with
methylprednisolone pulse therapy and /or intravenous
immunoglobulin (IVIG). Method: This prospective
observational study included children satisfying CDC MIS-C
criteria admitted from September to November, 2020. Primary
outcome was persistence of fever beyond 36 hours after start
of immunomodulation therapy. Secondary outcomes included
duration of ICU stay, mortality, need for repeat
immunomodulation, time to normalization of CRP and
persistence of coronary abnormalities at 2 weeks.
Results: Study population included 32 patients with
MIS-C with median (IQR) age of 7.5 (5-9.5) years. The
proportion of children with gastrointestinal symptoms was 27
(84%), cardiac was 29 (91%) and coronary artery dilatation
was 11 (34%). Pulse methylprednisolone and intravenous
immunoglobulin were used as first line therapy in 26 (81%),
and 6 (19%) patients, respec-tively. Treatment failure was
observed in 2/26 patients in methylprednisolone group and
2/6 patients in IVIG group. C-reactive protein levels less
than 60mg/L by day 3 was seen in 17(74%) in
methylprednisolone group and 2 (25%) in IVIG group (P=0.014).
There was no mortality. At 2 weeks follow-up coronary artery
dilatation persisted in 4 in methylprednisolone group and 1
in IVIG group. Conclusion: In patients with
SARS-CoV-2 related MIS-C, methylprednisolone pulse therapy
was associated with favorable short-term outcomes.
Keywords: Coronary artery, COVID-19, IVIG,
Kawasaki disease.
|
S
evere acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) related multisystem inflammatory
syndrome in children (MIS-C) is a dreaded complication that
is seen more often in children than in adults [1].
Intravenous immunoglobulin (IVIG) is considered as the
treatment of choice for Kawasaki disease (KD) [2]. MIS-C has
many dissi-milarities with KD, like occurrence in older
children (median age 10 years), presence of multi-organ
involve-ment, commonly gastrointestinal tract, myocar-dial
dysfunction and shock [3]. MIS-C has been treated
empirically with IVIG and steroids [2]. Some studies have
used biologicals like tumour necrosis factor inhibitor,
interleukin 1 inhibitor, interleukin 6 receptor antibody
etc. Most studies have used IVIG alone or in combination
with methylprednisolone than methyl-prednisolone alone in
the treatment of MIS-C [1,4]. Non-availability and high cost
of IVIG precludes its use in many centers. Hence, this
observational study was conducted to assess the clinical
profile and treatment outcome of patients treated with pulse
methyl-prednisolone.
METHODS
This observational study was conducted in
a tertiary care teaching hospital in India. This was the
preliminary analysis of an ongoing prospective observational
study at the institute. Ethics committee clearance was
obtained for the study and informed consent was taken from
patient caretakers. Children admitted with MIS-C aged 1
month to 12 years of age from September to November, 2020
were included.
Patients who fulfilled the CDC criteria
for diagnosis of MIS-C during the study period were included
in the study [5]. Infective causes like dengue,
leptospirosis, scrub typhus and bacterial sepsis were
excluded by appropriate investigations. SARS-CoV-2 reverse
trans-criptase polymerase chain reaction (RT-PCR) was done
in all patients, and SARS-CoV-2 antibody testing was done
using Vitros CoV2T kit [6].
Choice of immunomodulation was decided by
the treating unit based on patient demographics and Kerala
State guidelines for treatment of children with MIS-C [7].
Bedside echocardiography was done in all patients with MIS-C
with shock at admission. All patients were subsequently seen
by a pediatric cardiologist to look for coronary artery
status and cardiac dysfunction. Coronary artery diameter
z-score >2 was considered as coronary artery
dilatation/aneurysm [8]. Coronary artery changes like
increased echogenicity and non-tapering in the absence of
z-score >2 were taken as nonspecific coronary artery
changes. Shock was defined when a patient required more than
20 mL/kg of intravenous (IV) fluid resuscitation or
inotropic support to maintain blood pressure above the 5th
centile.
Study variables collected using
pre-designed pro-forma included patient demographic
characteristics, initial symptoms and clinical signs,
laboratory para-meters, type of immunomodulator used, time
to deferve-scence, duration of ICU stay, need for inotropic
support, duration of shock, duration and type of respiratory
support, coronary artery changes at admission and 2 weeks
follow-up and mortality. Patients who were treated with
methylprednisolone received pulse dose of 30 mg/kg once
daily for 3 days followed by oral prednisolone at 2 mg/kg
for 1 week or till CRP normalized, whichever was later.
Steroid was tapered and stopped over the next 2 - 3 weeks.
Children who were treated with IVIG received 2 g/kg as a
continuous infusion over 8-12 hours with longer duration in
patients with cardiac dysfunction.
Time to fever defervescence was recorded
at 12-hourly intervals. CRP and D-dimer were repeated on the
third and seventh day after the start of IVIG or
methylprednisolone. Treatment failure was defined as
persistence of fever or worsening of clinical condition
beyond 36 hours from the start of first-line therapy or
recrudescence of fever within 7 days. Repeat immuno-modulation
was considered if fever persisted beyond 36 hours of the
first dose of immunomodulatory therapy or if there was a
clinical deterioration, irrespective of time since finish of
first therapy. Children with treatment failure with IVIG
first dose were treated with a second dose of IVIG with
pulse methylprednisolone according to the Kerala State
guidelines [7]. Children with treatment failure with pulse
methylprednisolone were treated with IVIG. All patients were
followed up at two weeks after discharge.
All patients with shock were started on
low molecular weight heparin (LMWH) at prophylactic dose,
which was changed to treatment dose if thrombus was
detected. Children on LMWH were transitioned to low dose
aspirin once liver enzymes normalized and platelet count
increased to more than 80×10 9/L.
Children with thrombus were put on LMWH and anti-platelet
dose of aspirin. Anti-inflammatory dose of aspirin (50
mg/kg) was given in refractory MIS-C with KD like
presentation. Children receiving methylprednisolone also
received prophylactic IV pantoprazole.
Statistical analysis: Data
were entered in MS Excel and analyzed using SPSS 20.
Independent sample t test was used for comparison of
means. Categorical variables were compared using
nonparametric tests. Logistic regression was done to assess
the relationship between clinical variables and treatment
outcome.
RESULTS
A total of 32 (males, 21) patients with a
median (IQR) age of 7.5 (5-9.5) years were enrolled.
Seventeen patients were antibody positive, 8 patients were
both PCR and antibody positive, and two were only PCR
positive. Five patients were negative for PCR and antibody
but were epidemiologically related to COVID-19 positive
cases.
All children presented with fever with a
median (IQR) duration of 5 (3-6) days. The clinical
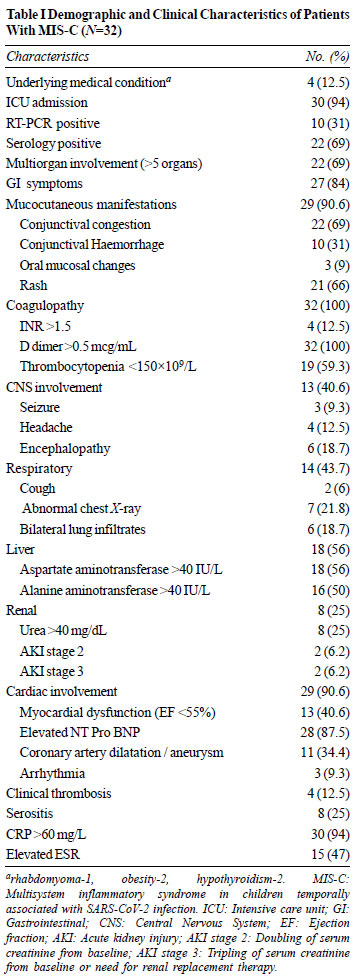
characteristics are shown in Table I. The mean
(SD) CRP was 141(72) mg/L and ESR 41(33.1) mm in the first
hour. The mean (SD) age of children with shock was
significantly higher than those without shock [7.93 (2.27)
vs 5.67 (3.39) years; P=0.02]. Children with shock
also had statistically significant higher D dimer [4.75
(3.3) vs 1.59 (0.982) mcg/mL; P=0.007], lower albumin
[2.8 (0.40) vs 3.32 (0.5) gm/dL, P=0.008], higher CRP
[152 (62.7) vs 120 (98.9) mg/L; P=0.049], higher
lactate [2.35 (1.27) vs 1.01(0.212) mmol/L; P=0.012]
and lower ejection fraction [53.5 (13.09) vs 65.1 (6.29)%;
P=0.015]. Eighteen patients (56%) had transaminitis
but hepatic failure was seen in only one child. Of the four
patients with vascular thromboembolic events (VTE), three
had thrombus in the left ventricle and one in the right
popliteal vein. Even though 10 (31%) patients were PCR
positive, antiviral therapy with remdesivir was offered only
to one child in our series.
 |
| |
Table II shows comparative clinical
features in children who received pulse methylprednisolone (n=26)
or IVIG (n=6). Treatment failure was observed in 2/26
patients in the methylprednisolone group and 2/6 patients in
the IVIG group. No child required additional immuno-modulation
with immune-biologicals or died during the study period.
Logistic regression was done to assess
the effect of clinical variables which were significantly
different between the two treatment groups, on the
likelihood of occurrence of treatment failure. Logistic
regression did not show any effect of age (P=0.7),
respiratory support (P=0.7) and five or more organ
involvement (P=0.2) on the likelihood of occurrence
of treatment failure.
Out of 11 patients with coronary artery
dilatation at admission, four had persistent dilatation at
two weeks. Six patients (21%) had echogenic non-tapering
coronaries but coronary artery diameter was less than 2 z-score.
One patient in this group developed coronary dilatation with
a z-score of more than 2.5 at 2 weeks. LV thrombus
had resolved in two patients at 2 weeks follow up while one
patient continued to have thrombus at 2 weeks follow up even
though the ejection fraction had normalized at 2 weeks. Of
the 13 patients with LV dysfunction, 11 (85%) had normal
ejection fraction at 2 weeks follow up. LV systolic function
normalized for the remaining 2 patients, at 6 weeks follow
up. One child had developed mononeuritis of the right
peroneal nerve after one week, which improved with the
continuation of steroids and aspirin at antiplatelet dose.
DISCUSSION
The present study reports favorable
outcomes in MIS-C with pulse methylprednisolone therapy.
MIS-C had dissimilarities to classical KD like higher age at
presentation and higher incidence of GI symptoms and shock,
as seen earlier [3,9,10].
Only 6% of children were referred with a
suspected diagnosis of MIS-C, highlighting the fact that
MIS-C continues to be a great masquerader. Clinical features
in children with acute SARS-CoV-2 infection included fever
in 49%, cough in 45% and GI symptoms in a few [11]. In
contrast, all children with MIS-C, had fever with a higher
proportion of GI symptoms, while cough was rare [12,14], as
also seen in the present study.
As previously reported, more children in
our study had conjunctival congestion than oral mucosal
changes [12,13]; 31% of children also had conjunctival
hemo-rrhage, which has not been reported in other studies.
Breathlessness was also observed in a higher proportion of
patients compared to cough [14]. Unilateral lung infiltrates
are more frequently reported in acute COVID-19 infection in
children [15]. While bilateral lung infiltrates were seen in
a higher proportion of patients with MIS-C.
A higher seropositivity rate with or
without SARS-CoV-2 RT-PCR positivity is reported in patients
with MIS-C with shock and multiorgan involvement [14].
Presence of positive COVID-19 antibody in patients with
positive SARS-CoV-2 PCR at admission probably indicates a
greater role of immune-mediated inflam-matory response than
acute SARS-CoV-2 viremia in the pathogenesis of MIS-C. As
many children with MIS-C have hepatic derangement, use of
antiviral therapy in these patients may be counterproductive
[13].
Cardiac involvement is the most
frequently reported organ dysfunction in MIS-C as also seen
in the present study [1,10,12,6]. Occurrence of coronary
artery aneurysm at follow up in a patient with nonspecific
coronary artery changes without dilatation in the initial
echocardiogram, highlights the need for meticulous follow up
with echocardiogram. Thrombosis has not been reported in
similar studies from India [16,17] but reported in studies
from the US and UK [12,13].
Earlier studies [12,14] have shown the
need for repeat IVIG and immunomodulators in almost 20% of
those who received IVIG. In our study, only 2 patients who
had received steroids subsequently needed IVIG. Logistic
regression did not show any relationship between clinical
variables like age, shock or multiorgan involvement with
initial treatment failure. None of the children required any
other alternative immunomodu-lators. There were no deaths or
need for ECMO in our study. Earlier studies have reported a
mortality of 1.2-2% [13,14] and need for additional cardiac
support with ECMO in 4% of patients [12,13].
Studies have reported favorable
short-term response to IVIG and steroid [3,14]. Currently
proposed treatment modalities are derived from its
similarity with KD and are based on expert opinion.
Treatment with IVIG in resource limited settings is a
challenge. In our study, children who received
methylprednisolone were significantly older and had a higher
number of organ involvement. Outcome measures showed a
favorable role for pulse methyl-prednisolone in the
treatment of MIS-C. A recent study [18] also found a more
favorable outcome in those treated with IVIG and
methylprednisolone than those treated with IVIG alone. Small
sample size, observational nature and absence of matched
cohorts are the main limitations of the study.
In patients with MIS-C with shock and
multi-organ dysfunction syndrome, IV methylprednisolone
pulse therapy was associated with favorable immediate and
short term follow-up outcomes. Patients with nonspecific
coronary changes like absence of tapering and increased
echogenicity need to be meticulously followed up for
occurrence of coronary artery dilatation even with a low
initial z-score.
Acknowledgements: Dr S Lakshmi, HOD
Pediatric Cardiology, Dr S Bindu, Unit Chiefs, Dr AS Ajith
Krishnan, Dr VH Sankar, Dr VK Devakumar and Dr Leela Kumari,
SAT, Government Medical College Thiruvananthapuram,
who were involved in patient care. Dr K Sarada Devi, HOD,
Department of Microbiology, Government Medical College
Thiruvanantha-puram and Dr Kavitha Raja, Professor,
Department of Microbiology, SCTIMST for their support and
guidance.
Ethics clearance: Human ethics
committee, Medical College, Thiruvnanthapuram; No.
01/32/2021/MCT, dated Jan 15, 2021.
Contributors: SS: conceptualized and
designed the study, analyzed data and participated in
manuscript writing. BS: statistical analysis and
interpretation of data, Critical revision of manuscript for
intellectual content, GS: Statistical analysis, drafting of
manuscript, Critical revision of manuscript for
intel-lectual content; NHR: acquisition, analysis and
interpretation of data, drafting of manuscript; SKA:
supervised the study and contributed to the critical
revision of manuscript for intellectual content. All authors
approve the final version of manuscript, and are accountable
for all aspects related to the study.
Funding: None; Competing interest:
None stated.
|
WHAT THIS STUDY ADDS?
•
Use of pulse methylprednisolone therapy as the
first line treatment for MIS-C was associated with
favorable immediate and short term follow up
outcomes.
|
REFERENCES
1. Verdoni L, Mazza A, Gervasoni A,
et al. An outbreak of severe Kawasaki-like disease at
the Italian epicentre of the SARS-CoV-2 epidemic: An
observational cohort study. Lancet. 2020;395:1771-8.
2. Jones VG, Mills M, Suarez D, et
al. COVID-19 and Kawasaki disease: Novel virus and novel
case. Hosp Pediatr. 2020;10:537-40.
3. Jiang L, Tang K, Levin M, et al.
COVID-19 and multisystem inflammatory syndrome in
children and adolescents. Lancet Infect Dis.
2020;20:e276-88.
4. Abrams JY, Godfred-Cato SE, Oster
ME, et al. Multisystem inflammatory syndrome in children
associated with severe acute respiratory syndrome
Coronavirus 2: A systematic review. J Pediatr.
2020;226:45-54.e1.
5. Centers for Disease Control and
Prevention. Multisystem inflammatory syndrome in
children (MIS-C) [Internet]. 2020. Accessed February 3,
2021. Available from: https://www.cdc.gov/mis-c/hcp/
6. Health C for D and R. EUA
Authorized Serology Test Performance. FDA [Internet].
Accessed February 26, 2021. Available from:
https://www.fda.gov/
medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
7. Government of Kerala.
Guidelines-Post-COVID-Clinics. pdf [Internet]. Accessed
February 1, 2021. Available from:
https://dhs.kerala.gov.in/wp-content/uploads/2020/10/
8. McCrindle BW, Rowley AH, Newburger
JW, et al. Diagnosis, Treatment, and Long-term
Management of Kawasaki Disease: A Scientific Statement
for Health Professionals from the American Heart
Association. Circulation. 2017;135:e927.
9. Bhat CS, Gupta L, Balasubramanian
S, et al. Hyperinflammatory syndrome in children
associated with COVID-19: Need for awareness. Indian
Pediatr. 2020;57:929-35.
10. Rowley AH, Shulman ST. The
epidemiology and pathogenesis of Kawasaki disease. Front
Pediatr [Internet]. 2018. Accessed December 7, 2020.
Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6298241/
11. Meena J, Yadav J, Saini L, et al.
Clinical features and outcome of SARS-CoV-2 infection in
children: A systematic review and meta-analysis. Indian
Pediatr. 2020;57:820-6.
12. Davies P, Evans C,
Kanthimathinathan HK, et al. Intensive care admissions
of children with paediatric inflammatory multisystem
syndrome temporally associated with SARS-CoV-2 (PIMS-TS)
in the UK: A multicentre observational study. Lancet
Child Adolesc Health. 2020;4:669-77.
13. Feldstein LR, Rose EB, Horwitz
SM, et al. Multisystem inflammatory syndrome in US.
Children and adolescents. N Engl J Med. 2020;383:334-46.
14. Godfred-Cato S, Bryant B, Leung
J, et al. COVID-19–associated multisystem inflammatory
syndrome in children — United States, March–July 2020.
MMWR Morb Mortal Wkly Rep. 2020;69:1074-80.
15. Kumar J, Meena J, Yadav A, Yadav
J. Radiological findings of COVID-19 in children: A
systematic review and meta-analysis. J Trop Pediat.
2020:fmaa045.
16. Dhanalakshmi K, Venkatraman A,
Balasubramanian S, et al. Epidemiological and clinical
profile of pediatric inflam-matory multisystem syndrome
- temporally associated with SARS-CoV-2 (PIMS-TS) in
Indian children. Indian Pediatr. 2020; 57:1010-14.
17. Jain S, Sen S, Lakshmi
venkateshiah S, et al. Multisystem inflammatory syndrome
in children with COVID-19 in Mumbai, India. Indian
Pediatr. 2020;57:1015-9.
18. Ouldali N, Toubiana J, Antona D,
et al. Association of intravenous immunoglobulins plus
methylprednisolone vs immunoglobulins alone with course
of fever in multisystem inflammatory syndrome in
children. JAMA.2021;325: 855-64.
19. Harwood R, Allin B, Jones CE, et
al. A National Consensus Management Pathway for
Paediatric Inflammatory Multisystem Syndrome Temporally
Associated With COVID-19 (PIMS-TS): Results of a
national Delphi process. Lancet Child Adolesc Health.
2021;5:133-41.
20. Shekerdemian LS, Mahmood NR,
Wolfe KK, et al. Characteristics and outcomes of
children with coronavirus disease 2019 (COVID-19)
infection admitted to US and Canadian pediatric
intensive care units. JAMA Pediatr. 2020;174:868-73.
21. Nakra NA, Blumberg DA,
Herrera-Guerra A, et al. Multi-system inflammatory
syndrome in children (MIS-C) following SARS-CoV-2
infection: Review of clinical presentation, hypothetical
pathogenesis, and proposed management. Children (Basel).
2020;7:E69.
22. Riphagen S, Gomez X,
Gonzalez-Martinez C, et al. Hyperinflammatory shock in
children during COVID-19 pandemic. Lancet.
2020;395:1607-8.
23. McCrindle Brian W, Rowley Anne H,
Newburger Jane W, et al. Diagnosis, treatment, and
long-term management of Kawasaki disease: A scientific
statement for health professionals from the American
Heart Association. Circulation. 2017 25;135:e927-99.
24. ToubianaJ, Poirault C, Corsia A,
et al. Kawasaki-like multisystem inflammatory syndrome
in children during the Covid-19 pandemic in Paris,
France: Prospective observational study. BMJ.
2020;369:m2094.
25. Dufort EM, Koumans EH, Chow E J,
et al; New York State and Centre for Disease Control and
Prevention Multi-system Inflammatory Syndrome in
Children Investigation Team. Multisystem inflammatory
syndrome in children in New York state. N Engl J Med.
2020;383: 347-58.
26. World Health Organization. Multisystem inflammatory
syndrome in children and adolescents temporally related to
COVID-19. Accessed August 27, 2020. Available from:
https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
|
|
|
 |
|

