|
|
|
Indian Pediatr 2021;58: 709-717 |
 |
Multicentric Hospital-Based Surveillance of
Pertussis Amongst Infants Admitted in Tertiary Care Facilities
in India
|
|
A Apte, 1 R Shrivastava,1
S Sanghavi,1 M Mitra,2
P Venkat Ramanan,3 J
Chhatwal,4 S Jain,1
J Chowdhury,2 S Premkumar,3
R Kumar,4 A Palani,3
G Kaur,4 N Javadekar,1
P Kulkarni,1 D Macina,5
A Bavdekar1
From Departments of Pediatrics, 1KEM Hospital Research
Centre, Pune; 2Institute of Child Heath, Kolkata; 3Sri Ramachandra
Medical Centre, Chennai; 4Christian Medical College and Hospital,
Ludhiana, India; and 5 Sanofi Pasteur, France.
Correspondence to: Dr Ashish Bavdekar, Associate Professor,
Consultant Pediatric Gastroenterologist, Department of Pediatrics, KEM
Hospital, Rasta Peth, Pune, Maharashtra 411 011.
Email:
[email protected]
Received: January 02, 2021;
Initial review: January 23, 2021;
Accepted: March 12, 2021.
CTRI Registration No: CTRI/2018/07/014911
|
|
Objective: To estimate the
disease and economic burden of pertussis amongst hospitalised infants in
India.
Design: Multicentric
hospital-based surveillance study.
Participants: Hospitalised
infants with clinical suspicion of pertussis based on predefined
criteria.
Outcome measures: Proportion of
infants with laboratory-confirmed pertussis, economic burden of
pertussis amongst hospitalised infants.
Results: 693 clinically suspected
infants were recruited of which 32 (4.62%) infants had
laboratory-confirmed pertussis. Progressive cough with post-tussive
emesis (50%) and pneumonia (34%) were the common clinical presentations;
apnea in young infants was significantly associated with pertussis.
Infants with pertussis were more likely to be younger (median age 102.5
days vs.157 days) and born preterm (42.9% vs 24.5%). Almost 30% infants
with pertussis had not received vaccine for pertussis with 50% of these
infants aged less than 2 months. Pertussis was associated with higher
costs of hospitalisation, pharmacy and loss of working days by
caregivers as compared to non-pertussis cases.
Conclusion: Younger infants,
those born preterm and those inadequately immunised against pertussis
are at higher risk of pertussis infection. Timely childhood immunisation
and introduction of maternal immunisation for pertussis can help in
reducing the disease burden.
Keywords: Bordetella pertussis, Burden,
Whooping cough.
|
|
D espite the introduction of
Diphtheria pertussis
tetanus (DPT) vaccine in the expanded
program on immunization, pertussis
(whooping cough) caused by Bordetella pertussis continues to be
an important public health problem with about 151000 cases reported
globally in 2018 [1]. According to a recent modelling study on the
global burden of pertussis, there are 5.1 million estimated pertussis
cases and 85900 estimated pertussis deaths amongst infants [2] and India
contributes to 26.7% (11,875 cases) of the global burden of pertussis
[3,4].
In the recent years, resurgence in pertussis has been
reported amongst infants and adolescents from many countries around the
world including United States, England [5,6], Brazil, Argentina [7] and
China [8]. The possible reasons for resurgence include waning immunity,
inadequate vaccine coverage, failure to administer booster doses after
the initial vaccination, differential herd immunity between whole cell
(wP) and acellular (aP) vaccines, diagnostic and epidemiologic
surveillance systems, and genetic changes in the pathogen [6,9,10].
Several hospital-based and community-based surveillance studies from
developed countries have reported a high rate of hospital-admissions due
to pertussis amongst infants, especially the youngest [11-13] and a
significant economic burden in infants hospitalised with complications
due to pertussis [14]. Maternal vaccination with the tetanus, diphtheria
reduced dose and acellular pertussis (Tdap) vaccine in third trimester
of pregnancy, neonatal vaccination, cocooning, adult and adolescent
immunization, addition of new antigens to the existing vaccine are some
of the strategies recommended towards reducing the resurgence of
pertussis [5,15].
A recent systematic review from Asia has highlighted
the burden of pertussis in neonates and the paucity of systematic data
in this regard [16]. In India, although the reported incidence of
pertussis has reduced significantly since 1987, due to lack of routine
laboratory diagnosis and uniformity in the clinical definition of
pertussis, large number of cases may go undetected and many
non-pertussis cases may be getting misdiagnosed as whooping cough
[4,17]. Thus, although India contributes significantly to global burden
of pertussis, country-specific estimates on the burden of pertussis in
infants at community or hospital level are not available, which are
important to inform the national immunization policy [17].
The national average for full immunization is only
62%, and nation-wide coverage for the 3rd primary dose of
DPT/pentavalent vaccine (containing DPT with H. influenzae B and
hepatitis B) is 78.4% as per National Family Health Survey-4 (NFHS-4)
[18]. In line with WHO recommendations, the public health programs in
India continue to use wP vaccines rather than aP vaccines [19];
although, the Indian Academy of Pediatrics recommends both wP and aP
vaccines for primary immunization [4,20]. The present study was designed
to estimate the disease and economic burden of pertussis amongst
hospitalized infants in a network of four tertiary care hospitals in
India.
METHODS
This cross-sectional, observational, multicentric
hospital- based active surveillance of pertussis was conducted in four
tertiary care hospitals in India – KEM Hospital, (KEMH) Pune,
Maharashtra; Sri Ramachandra Medical College, (SRMC) Chennai, Tamil
Nadu; Christian Medical College (CMC), Ludhiana, Punjab and Institute of
Child Health (ICH), Kolkata, West Bengal. The sites were chosen from
four different zones across the country to account for geographical,
seasonal and socioeconomic variations. The study was conducted from
October, 2018 to April, 2020 in the given four hospital sites.
The overall conduct of the multicentric study was
coordinated by a team of investigators and project managers at KEM
Hospital Research Centre, Pune (KEMHRC). This team was responsible
development of study protocol and study tools, training of all site
teams, site monitoring, data management and analysis. The sites teams
were trained for the study protocol, case record forms and
nasopharyngeal swab collection during investigators meetings arranged
before initiation of the study.
The study protocol was approved by the institutional
ethics committees of all the sites. The study was registered with
Clinical Trial Registry of India. The study participants were recruited
after obtaining written informed consent from their parents or
guardians.
The study was conducted in hospitalized infants with
clinical suspicion of pertussis. Three of the sites (KEMH, SRMC and ICH)
pre-screened potential study partici-pants from hospital registers
before screening them using the study criteria whereas at CMC, all
infants admitted were screened using study criteria. The clinical case
definition for pertussis was adapted from the criteria published by
Cherry, et al. [21], which were generally consistent with the case
definitions from the United States Centers for Disease Control and
Prevention (US CDC) [22], European Centre for Disease Prevention (ECDC)
[23] and World Health Organisation (WHO) [24].
A laboratory confirmed case (LCP) of pertussis was
defined as one with clinical criteria with at least one of the following
laboratory criteria: i) Detection of Bordetella pertussis,
Bordetella parapertussis or Bordetella holmesii nucleic acid
in a clinical specimen using real-time polymerase chain reaction
(RT-PCR); ii) Detection of B. pertussis or B.
parapertussis in a clinical specimen using culture.
For the enrolled infants, information on demography,
history of the present illness, vaccination records and socioeconomic
status was collected from the caregivers by trained clinical
coordinators on the case record forms at each site. The demographic
variables collected included gender, date of birth, birthweight,
gestational age and mode of delivery. Information on birthweight,
gestational age and anthropometric parameters was collected from the
hospitalisation records. The variables collected for vaccination status
included number of doses of DPT or pentavalent vaccine received, type of
vaccine (aP or wP) and the dates of vaccination. Details about the
onset, duration and clinical course of the current disease were
collected during the course of hospitalization till the child was
discharged/transferred from the inpatient facility.
Data about economic burden of the present illness at
household level were collected by interview method using a questionnaire
which included costs on use of health care resources (cost of
out-patient consultation, hospitalization, laboratory tests,
medications, physician/emergency room visits), use of non-health care
resources (travel, food and miscellaneous expenditure) and productivity
costs (loss of wages) for all clinically suspected cases for the given
episode of illness. Cost data towards management of pertussis-related
complications were collected till the end of present hospitalisation. In
addition, the socioeconomic status of the household was determined using
modified Kuppuswamy scoring [25]. The income of non-earning members of
the family e.g., housewives was assumed to be equivalent to minimum
daily wages of unskilled labour as per Government of India depending
upon their geographical area [26].
Two posterior nasopharyngeal swabs were collected by
trained clinical coordinators, nurse or laboratory technicians for all
children with clinical suspicion of pertussis, not later than 72 hours
following hospital admission and preferably before administration of
systemic antibiotics. The swabs were transported dipped in Amies medium
with charcoal/viral transport medium on dry ice in vaccine carrier to
the local microbiology laboratory.
In the local laboratory, the swabs for cultures were
immediately streaked on Bordet Gengou (BG) medium supplemented with 15%
defibrinated horse blood and containing cephalexin to inhibit normal
flora (40µg/mL). These culture plates were incubated for 7 days at
35-36°C and were inspected daily. Presence of any Bordetella
colonies were identified based on colony morphology, colony smear
showing Gram-negative coccobacilli and biochemical tests [27].
The swabs collected for RT-PCR were stored
immediately after collection for refrigeration at –20 oC
to -80oC till further
processing. The swabs were periodically (once in 2 months) shipped on
dry ice to microbiology laboratory, KEM Hospital, Pune for analysis of
RT-PCR (central laboratory). In the central laboratory, DNA was
extracted from the submitted specimens using a QIAamp DNA mini kit
(Qiagen) according to the manufacturer’s recommendations. The assays for
B. pertussis and B. parapertussis were done by RT-PCR
using Taqman technology for the amplification of the insertion elements
IS481 and IS1001 of Bordetella spp. Threshold cycle of
³35 was considered
positive for IS481. PCR assay for PtxA-S1 was carried out on all
specimens that tested positive for IS481 to confirm the diagnosis of
B. pertussis. Interpretation of results and identification of
species was done using WHO algorithm for diagnosis of pertussis [28] (Web
Table I).
During the study period, the central laboratory
completed a clinical proficiency testing program for B. pertussis
with Wisconsin State Laboratory Hygiene (WSLH), USA as a part of
external quality assurance.
Data management and analysis: At individual
sites, the data including clinical and laboratory data from the case
record forms were entered into a centrally managed electronic case
record forms generated using Open Clinica (community version). Source
data verification and quality control was managed by the central team at
KEMHRC. The anonymized dataset for the entire study was extracted for
analysis following source data verification. The dataset was archived at
local servers at KEMHRC.
The demographic factors were compared between
pertussis and non-pertussis cases. Young infants were defined as infants
with age <60 days [29]. Age-appro-priate pertussis vaccination was
defined as vaccination within 4 weeks of the exact age of eligibility
(i.e. for first dose of pertussis vaccine, vaccination within 10 weeks
of age is considered age appropriate). The proportion of
laboratory-confirmed pertussis was calculated and compared amongst
different age sub-populations (i.e. <2 months, 2-6 months, and
³6-12 months).
Occurrence of total cases and pertussis positive cases per month was
charted for total numbers as well as for site-specific cases.
Pearson Chi-square test was used for comparing the
proportions and Kolmogrov-Smirnoff test was used for comparison of
numerical data (non parametric data). All the analysis was done using
STATA 15.0.
RESULTS
A total of 916 infants were screened using clinical
case definition criteria, and 693 study participants were recruited (Fig.
1). Thirty two infants were detected with LCP; 8 from Pune, 17 from
Kolkata, 2 from Ludhiana and 5 from Chennai (Table I). The median
age of infants with LCP was about 3.6 months, and boys contributed 62.5%
cases of LCP (Table II).
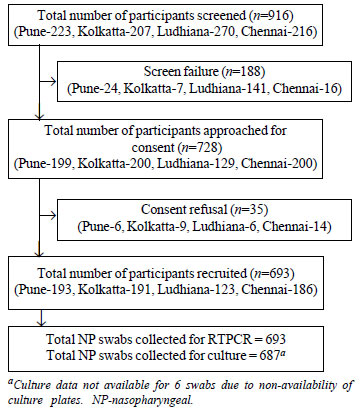 |
|
Fig. 1 Study flow chart.
|
Web Table II shows characteristics of study
participants recruited overall, and at each site. Approximately, two
third of the study participants were boys with a median age of 5 months.
About 85% of the participants were older than 2 months. Approximately,
one-fourth of the recruited children had low birthweight and/or were
born preterm.
About 75% of the study participants belonged to lower
or lower middle socioeconomic class as per modi-fied Kuppuswamy
classification. About 50% (n=355) of the study participants had
received age-appropriate vaccination for pertussis and 30% (n=214)
of them had received less than adequate vaccination. A total of 124
study participants had not received any pertussis vaccination of whom 81
were aged less than 2 months. Amongst these, 39 were aged less than 6
weeks and thus were not eligible to receive first dose of pertussis
vaccine.
Of the 687 cultures done, bacterial growth was
detected in 164 cultures. None of the 164 cultures grew Bordetella
species. Of 693 nasopharyngeal swabs collected for RT-PCR, Bordetella
species were detected in 32 (4.62%) swabs, of which 25 were B.
pertussis and 7 were B. parapertussis (Table I).
Presence of classical whoop was reported in only one
child. Apnea was significantly more associated with pertussis especially
in younger infants (aged <2 months). In addition to cough and fever, the
presenting symptoms for LCP included worsening of symptoms at night in
59%, post-tussive emesis in 50% and pneumonia in 34% children. Although
leukocytosis was reported in slightly higher proportion of children with
LCP, this difference was not statistically significant (Table II).
Infants with LCP were significantly younger than
those without LCP. Infants with LCP were more likely to have been born
preterm and were smaller in size. About 68% of infants with LCP were not
age-appropriately vaccinated for pertussis as compared to 48% of infants
without LCP. Amongst children aged less than 2 months, all the 5 cases
of LCP occurred in children who did not receive single dose of pertussis
vaccine and only one of these was aged less than 6 weeks and was thus
not eligible to receive first dose of pertussis vaccine (Table II).
There were no significant differences in the gender, birth weight or
socioeconomic status or receipt of antibiotic treatment in the two
groups.
Fig. 2 shows age-wise proportion of
laboratory-confirmed pertussis cases. In Pune and Chennai, the
proportion of LCP was higher in infants aged less than 2 months whereas
in Kolkata the highest number of cases was in the 2-6 months age group.
As a result, overall, there were more cases of LCP in the 2-6 months age
category as compared to less than 2 months and more than 6 months.
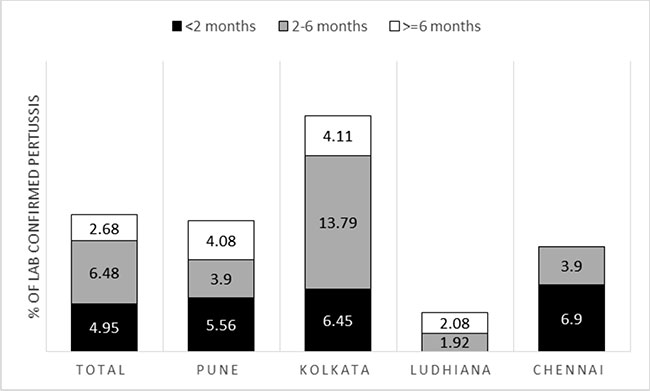 |
|
Fig. 2 Age-wise proportion of laboratory
confirmed pertussis cases.
|
Amongst the infants with LCP, 18% (n=6)
required ICU admission as compared to 23.6% (n=156) amongst
infants without LCP. Of these, only one infant with pertussis required
mechanical ventilation. The remaining 5 infants were treated with oxygen
therapy. Antibiotics were used in 23 infants, which mainly included
macrolides and cephalosporins.
There was complete recovery at the time of discharge
in 28 (87.5%) cases of pertussis. Two study participants with pertussis
had partially recovered at the time of hospital discharge without any
permanent debility and two children were discharged against medical
advice. There were no death during hospitalization amongst infants with
LCP as compared 8 deaths amongst infants without LCP.
Fig. 3 shows seasonal trends in occurrence of the
total cases and pertussis. Clinically suspected pertussis as well as LCP
cases were most frequent from October to January, which coincides with
winter season in India.
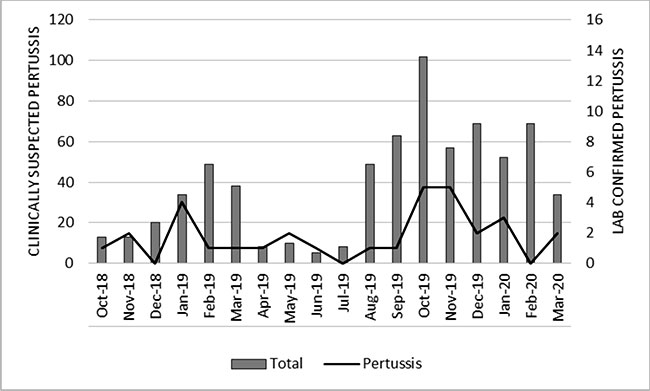 |
|
Fig. 3 Seasonality of occurrence of
pertussis.
|
Table III shows economic burden of pertussis at
household level. Hospitalization of an infant with LCP resulted in a
median hospitalization cost of approx. Rs.15000, median hospitalization
duration of 5.5 days and a median loss of worktime of 2 weeks by the
caregivers for taking care of the infant during the illness. This led to
median loss of income of Rs 6921 to caregivers of infants suffering from
LCP. The total cost for hospitalization including pharmacy cost was more
in infants with LCP. The days spent away from work by the caregivers
during illness were also significantly higher for LCP. For all the
infants with LCP, the families used their savings to meet the expenses
incurred. In addition, 15% families accepted donations from others and
28% families borrowed money to meet the expenses. It is noteworthy that
3% of the families had to sell their assets or use donations by
non-government organizations or hospitals to meet the hospitalisation
expenses.
DISCUSSION
This is the first hospital-based prospective
surveillance study for LCP amongst infants in India. The earlier
reported literature from India was an outbreak of suspected pertussis in
Arunachal Pradesh in 2007, with 71% of the suspected cases of pertussis
being under one year of age [30]. However, none of these children
underwent laboratory confirmation for pertussis. Although two
retrospective studies from tertiary care hospitals in India have been
reported recently with 30 and 36 cases of LCP in infants and children,
respectively; these studies present retrospective data from single
centers [31,32].
In our study, 4.62% of Indian infants hospitalized
with clinical suspicion of pertussis were found to have LCP. This is
much less than the numbers reported from hospital-based studies
conducted in Peru (39.5%) [12], Thailand (19%) [33], and a seven-country
multinational study including Brazil, Germany, Spain, Costa Rica,
Taiwan, Singapore and Uruguay (12%) [34]. A possible reason could be the
difference in the clinical definitions used for diagnosis of pertussis.
In the multinational study conducted by Kowalzik, et al. [34], infants
admitted in pediatric wards with any one of the clinical symptoms i.e.
respiratory failure, apnea, bradycardia, or cough accom-panied by
paroxysms, vomiting, whoop or cyanosis were included. Both the Thailand
[33] and the Peruvian [12] study used a clinical definition similar to
that of CDC [22]. However, the Thailand study recruited children
presen-ting to the outpatient clinic, whereas Peruvian study recruited
hospitalized children. In both these studies, children with chronic
respiratory or cardiac diseases were excluded. Two community-based
surveillance studies from other parts of South Asia have reported
relatively low incidence of pertussis amongst infants (13.3 and 3.96
cases per 1000 infant-years from Nepal [35] and Pakistan [36]).
Amongst the clinical features of LCP, progressive
cough with post-tussive emesis, pneumonia and worsening of symptoms at
night were common presenting features whereas classical whoop was found
in only one child with LCP. This highlights that inspiratory whoop,
which is mainstay of clinical diagnosis for pertussis in older children
and adults, may not present in infants [21]. Apnea and seizure were
presenting features in young infants with LCP but leukocytosis with
absolute lympho-cytosis was present only in one child aged less than 2
months. This is not consistent with Cherry, et al. [21] and other
hospitalized studies of pertussis [31,32,37,38] where leukocytosis with
absolute lymphocytosis was largely reported in young infants with
pertussis. Few studies have reported severe leukocytosis in critically
ill patients with pertussis [39,40]. Pneumonia was found in over 30% of
infants with LCP in our study; however, this is one of the many causes
of pneumonia. Overall, per-tussis contributes to only a fraction of
pneumonia hospitalizations amongst infants from low- and middle-income
countries [38,41]. These observations point towards equivocality of
clinical criteria and need for more frequent laboratory diagnosis of
pertussis amongst children.
Almost 75% of the infants with LCP were aged less
than 6 months and 15% were aged less than 2 months in our study.
Retrospective studies from Indonesia [42], Philippines [38,43] and
Singapore [37] have also reported pertussis cases with higher occurrence
and mortality in infants aged less than 6 months. Bhattacharya, et al.
[31] reported about 60% of cases in infants aged less than 16 weeks and
30% cases in infants aged less than 8 weeks. Our findings emphasize the
earlier observation that pertussis can present with severe morbidity in
younger infants requiring hospitalisation [40]. However, only 18-20% of
our study participants with LCP required admissions in the intensive
care and only one child required mechanical ventilation as against
substantial morbidity and mortality reported from studies done elsewhere
[37,38,40,42]. Children born as preterm presented as an additional risk
factor for pertussis which has also been reported earlier [44]. This
could partly be due to delay in the vaccination for preterm children
(46.9% full vaccination in preterm as compared 54% amongst others).
In our study, inadequate vaccination or delayed
vaccination for pertussis was found to be an important risk factor.
Almost 30% infants with LCP had not received vaccine for pertussis, 50%
of these infants aged less than 2 months. Another 30% had received less
than adequate pertussis vaccination. Similar results were reported in
earlier retrospective Indian study conducted by Kavita, et al. [32] and
a recently conducted Chinese study by Wang, et al. [40]. Lack of timely
vaccination has been reported to be an important preventable risk factor
for pertussis amongst young infants globally [16,43,45] not only as a
direct risk from lack of protection but also indirectly as infected
young infants and children can contribute to increase circulation and
cause infection of infants who are too young to get vaccinated and but
at high risk of developing complications due to pertussis. This Indian
scenario is different from the Western world where resurgence of
pertussis has been documented despite high coverage of childhood
pertussis immunisation and where the main postulated cause of pertussis
is reported to be waning immunity from childhood vaccine in mothers
[46]. Introduction of maternal immunization with TdaP has been shown to
protect young infants from pertussis and can be useful strategy in our
setup as well [15].
Majority of the infants in our study had received wP
vaccine and only 4-6% of them received aP vaccine for their primary
immunization. National immunization program in India continues to use wP
based on the WHO recommendations to continue wP vaccine in countries
where it is part of the program in order to minimise the risk of
pertussis resurgence associated with aP vaccines [45].
The costs associated with LCP were higher than that
of non-LCP due to increased hospitalization and pharmacy costs. As
almost 75-80% of the families belonged to lower or lower-middle
socioeconomic status, the hospitalization posed significant economic
burden on the households leading to stretching of the existing
resources. Thus, 3% of the families had to resort to selling their
assets or borrowing to meet the expenditure.
Our study has few limitations. Since it only focused
on the hospitalized cases of pertussis, children admitted in the day
care centres or visiting outpatient departments of the tertiary care
centres with similar symptoms were not recruited. The clinical outcome
after hospital discharge was not monitored. Although the nasopharyn-geal
swabs were collected within 72 hrs of hospitalization and preferably
before administration of antibiotics, large proportion of the infants
had received antibiotics before hospitalization. Although uniform
clinical criteria were used for identification of clinically suspected
pertussis cases, one of the study sites did not pre-screen potential
study participants giving rise to higher screen failures as compared to
the other three sites. The study did not collect information about
household contacts for pertussis and does not provide population-based
incidence of pertussis. None-the-less, the study emphasizes increased
risk of pertussis amongst young Indian infants, especially those not
fully vaccinated.
Our study provides the first systematic evidence for
burden of pertussis amongst hospitalized infants in India. Younger
infants, those born preterm and inadequately immunized against pertussis
are at higher risk of infection. Efforts to reduce delay in primary
immunization and introduction of maternal immunization for pertussis can
clearly help in reducing the disease burden in young infants.
Acknowledgements: Sonali Shah from KEM Hospital
Research Centre and Kapil Bhagat from Christian Medical College for
their assistance in clinical data acquisition. Tathagata Bhattacharjee
for handling data management for all the four sites.
Ethics clearance: Institutional Ethics Committee
of KEM Hospital Research Centre, Pune No. 1801, dated June 30, 2018.
Contributors: AA, SS: concept or design,
supervision, data analysis and interpretation, drafting publication,
critical revision, final approval, accountable for accuracy and data
integrity; RS: data acquisition, project management, drafting
publication, critical revision, final approval, accountable for accuracy
and data integrity; MM,PVR,SJ: data acquisition, data interpretation,
critical revision, final approval, accountable for accuracy and data
integrity; JC: data acquisition, supervision, data interpretation,
critical revision, final approval, accountable for accuracy and data
integrity; JC,SP,AP: data acquisition, critical revision, final
approval, accountable for accuracy and data integrity; RK, GK: data
acquisition, supervision, critical revision, final approval, accountable
for accuracy and data integrity; NJ: data acquisition, data analysis and
interpretation, critical revision, final approval, accountable for
accuracy and data integrity; PK: design or concept, critical revision,
final approval, accountable for accuracy and data integrity; DM: design
or concept, funding acquisition, critical revision, final approval,
accountable for accuracy and data integrity; AB: design or concept,
supervision, funding acquisition, critical revision, final approval,
accountable for accuracy and data integrity. All authors approved the
final version of manuscript, and are accountable for all aspects related
to the study.
Funding: This project was funded by Sanofi
Pasteur, India (Grant number: PER0062).
Competing interest: Denis Macina is
currently employed by Sanofi Pasteur SA and also reports holding of
shares in the Sanofi group of companies as part of his employee
remuneration. All other authors declare no competing interests.
|
WHAT IS ALREADY KNOWN?
•
Pertussis can lead to severe manifestations in infants
requiring hospitalization.
WHAT THIS STUDY ADDS?
• Laboratory confirmed
pertussis was seen in 4.6% of children hospitalized with a
clinically diagnosed pertussis.
•
Younger age, prematurity and inadequate immunization against
pertussis were the major risk factors for pertussis.
|
REFERENCES
1. World Health Organisation. Pertussis 2018.
Available from:https://www.who.int/health-topics/pertussis#tab
=tab_1. Accessed August 28, 2020.
2. Yeung KHT, Duclos P, Nelson EAS, Hutubessy
RCW. An update of the global burden of pertussis in children younger
than 5 years: a modelling study. Lancet Infect Dis. 2017;
17:974-80.
3. WHO. WHO vaccine-preventable diseases:
monitoring system 2020 global summary. 2020. Accessed August 28,
2020. Available from: https://apps.who.int/immunization_
monitoring/globalsummary/countries?countrycriteria %5Bcountry %5D%5
B%5D=IND& commit=OK.
4. Dewan P, Shah D. Pertussis:100-day disease
over 50 years! Indian Pediatr. 2019;56:865-7.
5. Burns DL, Meade BD, Messionnier NE. Pertussis
resurgence: perspectives from the Working Group Meeting on pertussis
on the causes, possible paths forward, and gaps in our knowledge. J
Infect Dis. 2014;209:S32-5.
6. Ausiello C, Cassone A. Acellular Pertussis
Vaccines and Pertussis Resurgence: Revise or Replace? mBio. 2014;5.
7. Hozbor D, Ulloa-Gutierrez R, Marino C, et al.
Pertussis in Latin America: Recent epidemiological data presented at
the 2017 Global Pertussis Initiative meeting. Vaccine.
2019;37:5414-21.
8. Zhang Y, Bambrick H, Mengersen K, et al.
Resurgence of Pertussis Infections in Shandong, China: Space-Time
Cluster and Trend Analysis. Am J Trop Med Hyg. 2019;100:1342-54.
9. Torres RSLA, Santos TZ, Torres RAA, et al.
Resurgence of pertussis at the age of vaccination: clinical,
epidemio-logical, and molecular aspects. J Pediatr. 2015;91:333-8.
10. Lapidot R, Gill CJ. The Pertussis resurgence:
putting together the pieces of the puzzle. Trop Dis Travel Med
Vaccines. 2016;2:26.
11. Rendi-Wagner P, Kundi M, Mikolasek A, et al.
Hospital-based active surveillance of childhood pertussis in Austria
from 1996 to 2003: Estimates of incidence and vaccine effectiveness
of whole-cell and acellular vaccine. Vaccine. 2006;24:5960–5.
12. Castillo ME, Bada C, del Aguila O, et al.
Detection of Bordetella pertussis using a PCR test in infants
younger than one year old hospitalized with whooping cough in five
Peruvian hospitals. Internat J Infect Dis. 2015;41:36-41.
13. Crowcroft NS, Booy R, Harrison T, et al.
Severe and unrecognised: pertussis in UK infants. Arch Dis
Childhood. 2003;88:802-6.
14. Greenberg DP, Caro JJ. Summary Health and
economic burden of pertussis. Pediatr Infect Dis J. 2005;24:S55-7.
15. Bento AI, King AA, Rohani P. Maternal
pertussis immunisation: Clinical gains and epidemiological legacy.
Euro Surveill. 2017;22:30510.
16. Agrawal A, Singh S, Kolhapure S, et al.
Neonatal Pertussis, an Under-Recognized Health Burden and Rationale
for Maternal Immunization: A Systematic Review of South and
South-East Asian Countries. Infect Dis Ther. 2019;8:139-53.
17. Chitkara AJ, Kukreja S, Shah RC. Pertussis
and diphtheria immunization. Indian Pediatr. 2008;45:723-7.
18. International Institute for Population
Sciences. National Family Health Survey (NFHS-4). 2016.
19. Arciniega J, Corbel M, Gaines-Das R, et al.
Recommendations for whole-cell pertussis vaccine. World Health
Organization - Technical Report Series. 2007; 941:301-33.
20. Balasubramanian S, Shah A, Pemde HK, et al.
Indian Academy of Pediatrics (IAP) Advisory Committee on Vaccines
and Immunization Practices (ACVIP) Recommended Immunization
Schedule (2018-19) and Update on Immunization for Children Aged 0
Through 18 Years. Indian Pediatr. 2018;55:1066-74.
21. Cherry JD, Tan T, Wirsing von König C-H, et
al. Clinical definitions of pertussis: Summary of a Global Pertussis
Initiative roundtable meeting, February 2011. Clin Infect Dis.
2012;54:1756-64.
22. Centers for disease control and prevention.
Pertussis (Whooping Cough) (Bordetella pertussis) - 2014 Case
Definition. Accessed August 28, 2020. Available from:
https://wwwn.cdc.gov/ nndss/ conditions/pertussis/case-definition/2020
23. Union E, Area EE, Centre E, Prevention D.
Expert consultation on pertussis 1 Background 2 Session I/ : Is
pertussis an issue in the EU/ ? Vol. 375. 2012. Accessed March 03,
2021. Available from: https://www.ecdc.
europa.eu/sites/portal/files/media/en/publications/Publi cations/pertussis-meeting-2012.pdf
24. World Health Organization (WHO). Pertussis
Vaccine-Preventable Diseases. Accessed March 03, 2021. Available
from:https://www.who.int/immunization/monitoring_sur veillance/burden/vpd/WHO_SurveillanceVaccine
Preventable_16_ Pertussis_ R1.pdf?ua=1
25. Saleem SM. Modified Kuppuswamy scle updated
for the year 2018. Paripex - Indian J Res 2018;7:217-8.
26. Ministry of Labour and Emoyment, Government
of India. Chief Labour Commissioner (Central). 2018. Accessed March
03, 2021. Available from: https://clc.gov.in/clc/node/586
27. Lee AD, Cassiday PK, Pawloski LC, et al.
Clinical evaluation and validation of laboratory methods for the
diagnosis of bordetella pertussis infection: Culture, polymerase
chain reaction (PCR) and anti-pertussis toxin IgG serology (IgG-PT).
PLoS One. 2018;13:1-20.
28. World Health Organisation. Laboratoy Manual
for the diagnosis of Whooping cough caused by Bordetella pertussis
and bordetella parapertussis- Update 2014. Accessed March 03, 2021.
Available from: www.who.int/vaccines-documents/%0 ACopies
29. Roy S, Patil R, Apte A, et al. Feasibility of
implementation of simplified management of young infants with
possible serious bacterial infection when referral is not feasible
in tribal areas of Pune district, Maharashtra, India. PLoS One.
2020;15:e0236355.
30. Takum T, Gara D, Tagyung H, Murhekar MV. An
outbreak of pertussis in Sarli Circle of Kurung-kumey district,
Arunachal Pradesh, India. Indian Pediatr. 2009;46:1017-20.
31. Bhattacharya D, Dash N, Kavitha TK, Sharma M,
Gautam V, Verma S. Lurking infantile pertussis: experience from a
tertiary care center in Northern India. J Pediatr Infect Dis.
2020;15:257-61.
32. Kavitha TK, Samprathi M, Jayashree M, Gautam
V, Sangal L. Clinical profile of critical pertussis in children at a
pediatric intensive care unit in Northern India. Indian Pediatr
2020;57:228-31.
33. Suntarattiwong P, Kanjanabura K, Laopipattana
T, et al. Pertussis surveillance in a children hospital in Bangkok,
Thailand. Internat J Infect Dis. 2019;81:43-5.
34. Kowalzik F, Barbosa AP, Fernandes VR, et al.
Prospective multinational study of pertussis infection in
hospitalized infants and their household contacts. The Pediatr
Infect Dis J. 2007;26:238-42.
35. Hughes MM, Englund JA, Kuypers J, et al.
Population-based pertussis incidence and risk factors in infants
less than 6 months in Nepal. J Pediatr Infect Dis Soc. 2017;6:33-9.
36. Omer SB, Kazi AM, Bednarczyk RA, et al.
Epidemiology of pertussis among young pakistani infants: A
community-based prospective surveillance study. Clin Infect Dis.
2016;63:S148-53.
37. Chong CY, Yung CF, Tan NWH, Acharyya S, Thoon
KC. Risk factors of ICU or high dependency requirements amongst
hospitalized pediatric pertussis cases: A 10 year retrospective
series, Singapore. Vaccine. 2017;35:6422-8.
38. Sadiasa A, Saito-Obata M, Dapat C, et al.
Bordetella pertussis infection in children with severe pneumonia,
Philippines, 2012–2015. Vaccine. 2017;35:993-6.
39. Ganeshalingham A, McSharry B, Anderson B,
Grant C, Beca J. Identifying children at risk of malignant
bordetella pertussis infection. Pediatr Crit Care Med. 2017;18.
40. Wang C, Zhang H, Zhang Y, et al. Analysis of
clinical characteristics of severe pertussis in infants and
children: a retrospective study. BMC Pediatr. 2021;21:65.
41. Barger-Kamate B, Knoll MD, Kagucia EW, et al.
Pertussis-associated pneumonia in infants and children from low-and
middle-income countries participating in the perch study. Clin
Infect Dis 2016;63(Suppl 4):S187-96.
42. Nataprawira HM, Phangkawira E. A
retrospective study of acute pertussis in Hasan Sadikin
Hospital–Indonesia. J Acute Dis. 2015;4:147-51.
43. Bonus RBF, delos Reyes CA, Dy CAME, Ramos RA.
Clinical profile of pertussis among pediatric patients admitted at
the Philippine General Hospital. Pediatr Infect Dis Soc Philippines
J. 2015;16:21-7.
44. Riise ØR, Laake I, Vestrheim D, et al. Risk
of Pertussis in Relation to Degree of Prematurity in Children Less
Than 2 Years of Age. Pediatr Infect Dis J. 2017;36:e151-6.
45. World Health Organization. Pertussis
vaccines: WHO position paper - August 2015. Weekly Epidemiological
Record. 2015;90:433-60.
46. Torres RSLA, Santos TZ, Torres RAA, et al. Resurgence of
pertussis at the age of vaccination: Clinical, epidemio-logical, and
molecular aspects. J Pediatr. 2015;91:333-8.
|
|
|
 |
|

